In the present investigation we ascertained the stability of lycopene, ß-carotene, ascorbic acid, polyphenolic compounds and total antioxidant capacity (AC) during the process of concentrating tomatoes into two tomato pastes (10 and 15ºBrix). Thermal processing increased the content of lycopene, total phenolic compounds, total flavonoids, and the individual phenolic compounds quercetin, rutin, chlorogenic and cafeic acids, whereas it decreased the other analysed compounds. However, lycopene in the 15ºBrix-tomato paste decreased due to the extension of thermal processing, which led to degradation. The AC of aqueous and organic extracts was measured and different AC values were observed depending on the antioxidant profile of the extract and assay used (TEAC and FRAP). AC expressed in dry matter decreased as result of ascorbic acid losses. Overall, thermal processing enhanced the nutritional value of tomatoes, mainly by increasing the lycopene and phenolic antioxidants, but the extension of treatment must be controlled to prevent lycopene degradation.
Key words: Tomato; lycopene, β-carotene, phenolic compounds, ascorbic acid, antioxidant capacity, thermal processing.
En el presente trabajo hemos estudiado la estabilidad del licopeno, β-caroteno, ácido ascórbico, compuestos fenólicos y capacidad antioxidante total (AC) durante el procesado de concentración del tomate en dos pastas de tomate (10 y 15ºBrix). El tratamiento térmico incrementó el contenido de licopeno, compuestos fenólicos totales, flavonoides totales y el contenido de quercetina, rutina y ácido clorogénico y cafeíco, disminuyendo el contenido de los otros compuestos analizados. Sin embargo, el contenido de licopeno en la pasta de tomate de 15ºBrix disminuyó debido al tratamiento térmico como consecuencia de la degradación térmica. La AC de los extractos acuosos y orgánicos de las muestras proporcionaron diferentes resultados dependiendo del perfil de antioxidante extraído y del método de análisis utilizado (TEAC y FRAP). La AC expresada en material seca disminuyó como resultado de las pérdidas de ácido ascórbico. En general el procesado térmico incrementa el valor nutricional del tomate , debido principalmente al incrmento de licopeno y compuestos fenólicos, pero la extensión del tratamiento en tiempo y temperatura debe ser controlado para prevenir la degradación del licopeno.
Palabras clave: Tomate, licopeno, β-caroteno, compuestos fenólicos, ácido ascórbico, capacidad antioxidante, procesado térmico.
Departamento de Tecnología de los Alimentos, Nutrición y Bromatología. Facultad de Veterinaria de la Universidad de Murcia, Murcia, España
Tomatoes are one of the most important commercial vegetable crops in the world. Epidemiological studies describe an inverse relation between a diet rich in tomatoes and tomato products and the incidence of cardiovascular disease and several types of cancer (1, 2). This protective effect has been attributed to their high content of various dietary compounds, e.g., carotenoids and polyphenolic compounds, vitamin E, potassium and selenium, which exert different bioactive properties.
Lycopene is the predominant carotenoid of tomatoes, and tomatoes are its main dietary source (1). Also, β-carotene is present, although in minor concentrations. Furthermore, tomatoes are a good source of polyphenolic compounds, such as flavonoids and hydroxycinnamic acids (3,4), and vitamin C (5).
Data from the EPIC-study show that the intake of tomatoes and tomato products in Europe ranges from an average of 15.7 g/d in The Netherlands to 97.6 g/d in the south of Spain to 163.6 g/d in Greece. The intake of raw tomato was generally higher than that of cooked tomatoes (6). This is in contrast to consumption data obtained in the United States, where most tomatoes consumed are processed (89.8 g/d versus 21.7 g/d for raw tomatoes, data adapted from Willcox (2).
It is well known that processing tomatoes (using heat and mechanical treatment) releases lycopene from the food matrix and results in a better bioavailability of lycopene (7,8). However, processing might have a detrimental effect on other micronutrients, especially on the heat-sensitive vitamin C (9,5). Investigations into the effect of food processing (post-harvest storage, thermal treatment and storage during shelf-life) on the ensemble of naturally occurring antioxidants and its impact on the total antioxidant capacity of tomato products have been carried out considering the effect on carotenoids, vitamin C and/or total phenolic compounds (5,9-19). However, none of these studies have jointly considered the effect of food processing on the isomerization of carotenoids, ascorbic acid content, changes in individual phenolic compounds, and antioxidant activity.
The aim of the present investigation was to ascertain the stability of lycopene, β-carotene, ascorbic acid, and major individual phenolic compounds during the process of concentrating tomatoes into tomato paste. In addition, the relationship between the antioxidant compounds and antioxidant activity was also evaluated.
Tomatoes (Lycopersicon esculentum), category “Extra”, size III, stage 6 (bright red) of variety “pera” were purchased in a local supermarket. Raw tomato (RT, 4 ºBrix) was homogenised and concentrated to obtain two types of tomato paste. The concentration process was carried out in the pilot plant of the department of Food Technology, Food Science and Nutrition using a Thermomix-21 (Vorwerk Spain, Madrid). The two tomato pastes were TP1, with 10 ºBrix (110 ºC for 15 min), and TP2, with 15 ºBrix (110 ºC for 30 min).
Total solids (TS) and moisture (M) were determined by oven-drying the samples at 105 ºC to a constant weight. Soluble solids (SS) were quantified at 20°C using a Leica Abbe Mark II Refractometer (Buffalo, NY, USA). The colour readings were taken for each sample using a Minolta Chromameter Reflectance II CR-2000 (Minolta Limited, Milton Keynes, UK). The values a* (red-green) and yellow (yellow-blue) were used to calculate a*/b* ratio (Descriptive data are shown in Table 1).
Carotenoids were determined using HPLC as described previously (16,20). To approx. 2 g of sample, 350 mg MgO and internal standard apo-β-8´-carotenal were added. Samples were extracted thrice with 35 ml of methanol/tetrahydrofuran (1:1, v/v) containing 0.1% butylated hydroxytoluene (BHT) as an antioxidant and the extracts were dried on a rotary evaporator. The residue was dissolved in methanol/tetrahydrofuran (1:1, v/v, without BHT) before injected in the HPLC (Labchrom Merck-Hitachi system) with a C30-column (Trentec, 250 x 4.6 mm, 5 μm) and a diode array detector (DAD). The analysis ran at 17 ºC with the DAD set at 450 nm and 472 nm to record chromatograms, using methanol and methyl tert-butyl ether as mobile phases. The carotenoid content of the samples was quantified by comparing peak areas to those of authentic standards (lutein, (all-E)-β-carotene, (13Z)-β-carotene and (all-E)-lycopene). Amounts of lycopene (Z)-isomers were quantified by comparing the area to that of (all-E)-lycopene.
For analysis of TPC and TF 2 g of tomato samples were homogenised with 80% (v/v) aqueous methanol containing 1% HCl and shaking for 2 h at room temperature. Samples were centrifuged at 3500 rpm for 15 min, and 1 ml of each supernatant was taken to develop colorimetric reactions. TPC was determined photometrically using Folin-Ciocalteau reagent using gallic acid monohydrate (Sigma, St. Louis, USA) as the standard. TPC content was expressed as mg gallic acid equivalents (GAE) per 100 g of tomato (9). For TFl was quantified photometrically as described Dewanto et al. (12) using (+)-Catehchin (Sigma, St. Louis, USA) as the standard. TFl content was expressed as mg catechin equivalents (CE) per 100 g of tomato (12).
Flavonoids were determined by HPLC following the hydrolysis method described by Crozier (21) and Martínez-Valverde (3). The flavonoids investigated were: quercetin, rutin, kaempferol and naringenin; and the hydroxycinnamic acids were: ferulic acid, cafeic acid, chlorogenic acid and p-coumaric acid. Flavonoids and hydroxycinnamic acids were analyzed using the same HPLC method. An HPLC system with a DAD detector (Labchrom Merck-Hitachi system) and an RP C18-column (Lycrosorb, 250 x 4.6 mm, 5 μm) was set at 35 °C. Water acidified with 0.05% triflouracetic acid (TFA) and methanol were used as mobile phases. The compounds were quantified by comparing the areas to those of authentic standards (Sigma Chemical, St. Louis, USA).
Ascorbic acid was determined by HPLC using an RP C18-column (Lycrosorb, 250 x 4.6 mm, 5μm) (Labchrom Merck-Hitachi system) according to the method described by Esteve (22).
Four tomato extracts (aqueous, 5 mM phosphate buffer solution, pH 7.4, methanol and ethanol) were prepared by blending approx. 4 g of sample with 10 ml of the respective solvents for 5 min. Then samples were centrifuged (10000 rpm, 5 min) and supernatants were assayed using the TEAC () and FRAP (ferric reducing ability) methods.
Trolox equivalent antioxidant capacity (TEAC): 1.0 ml of ABTS•+ radical cation solution and 100 μl of sample were mixed vigorously, and absorbance was read at 734 nm exactly 2 min after initiation of the reaction. Results were expressed as mM Trolox equivalents (TE) (23, 24).
Ferric reducing ability (FRAP): The FRAP reagent was prepared as described Benzie and Strain (25) and were mixed with 25 μl of sample and with 75 μl of water. The reaction was monitored for as long as 4 min. The absorbance readings at this time (4 min) were used to calculate the FRAP values. Aqueous solutions of known Fe2+ concentration in the range of 100 to 2000 μM (FeSO4·7H2O) were used for calibration, and the results were expressed as μM of Fe2+ equivalents. One Fe2+ equivalent per litre equals the amount of Fe2+ per litre required to give the same absorbance change (25).
Statistical analysis of the data was performed using SPSS modules, Windows version 14.0 (SPSS Inc, Chicago, IL). An analysis of variance (one-way-ANOVA) was included in the data treatment to study the effect of thermal treatment on the individual antioxidants and total antioxidant capacity of tomato and tomato pastes. Tukey’s test for pairwise comparison was used to determine significant differences at the 5% level. Pearson correlation and linear regression were carried out to understand any relation among the analysed parameters.
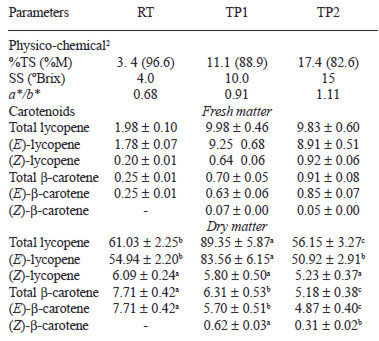
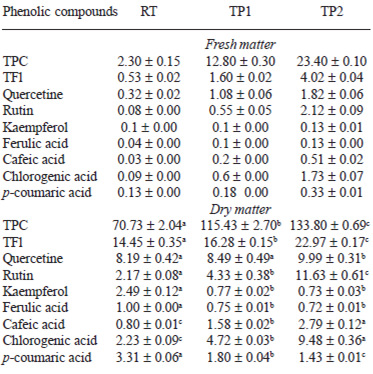
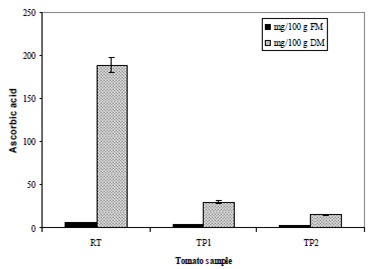
Table 1 shows the physico-chemical parameters of the RT and both tomato pastes (TP1 and TP2). The concentration process led to an increase in TS, SS and redness, as determined by the ratio a*/b*. In a general view, the effect of thermal treatment on the antioxidant compounds was related to the loss of water content in the samples and the extension of thermal processing. The concentration process reduced the water content in the samples and consequently, the carotenoid content (Table 1); TPC and TFl increased significantly with thermal treatment (Table 2). On the contrary, ascorbic acid decreased from RT to the tomato pastes (TP1 and TP2) (Figure 1). Different behaviour was observed in the content of these compounds when the results were expressed as dry matter, as shown below.
RT contained about 1.98 mg lycopene/100 g fresh matter, as measured by HPLC. Heat treatment resulted in a concentration of lycopene to 9.89 mg/100 g fresh matter and 9.83 mg/100 g of fresh matter, in TP1 and TP2, respectively. With regard to calculations with dry matter, a significant increase in lycopene occurred in the processing step from RT to TP1, but with further processing to TP2, lycopene concentration decreased significantly, about 38%, to a concentration comparable to RT (Table 1). Similar behaviour was observed in the content of all-(E)-lycopene, which composed about 90% of the total lycopene present in RT, TP1 and TP2 (Table 1). Lycopene was not susceptible to thermal isomerization; isomeric distribution was not altered during heat treatment. (Z)-lycopene ranged between 6.5% and 10%, being the main isomer of (15Z)-lycopene in the (Z)-configuration. All three samples contained β-carotene in concentrations about 10-fold less than that of lycopene. TP1 and TP2 showed a higher content of β-carotene than RT expressed as fresh matter (Table 1). However when data were expressed as dry matter, a significant reduction was observed in the total and all-(E)- β-carotene content. As the time of thermal treatment was extended, the degradation of β-carotene increased, with values of 7.71 mg/100 g of dry matter in RT and 5.18 mg/100 g of dry matter in TP2. The thermal treatment also caused isomerization of β-carotene; (Z)-isomers were not detected in the raw sample, but 9.8 % and 6% of (Z)- β-carotene appeared in the heat-treated samples TP1 and TP2, respectively (Table 1). Lutein was not found in the samples.
TPC increased in fresh matter from 2.30 mg GAE/100 g in RT to 12.80 and 23.40 mg GAE/100 g in TP1 and TP2, respectively (Table 2). In addition, when considering dry matter, heat treatment resulted in a significant increase of TPC of 63% and 89% in TC1 and TC2, respectively. The same behaviour was observed in the content of TFl expressed for both fresh and dry matter (Table 2). TF was concentrated significantly as a function of the extension of treatment in fresh matter from 0.53 mg of CE/100 g in RT to 4.02 mg of CE/100 g in TP2 (Table 2), resulting also in a significant increase, up to 60% in TP2, when data are expressed for dry matter. Hence, interestingly, the perceptual fraction of TFl relative to TPC increased from 9.3% in RT to 11.0% and 16.1% in TP1 and TP2, respectively (data not shown).
Heat treatment increased the content of individual flavonoids and hydroxycinnamic acids expressed as fresh matter (Table 2). However, in dry matter the behaviour differed depending on the compound. Kaempferol content decreased significantly from RT to both tomato pastes, which showed a similar final content of around 0.77 mg/100 of dry matter (Table 2). Although naringenin was analysed using HPLC, it was not detected in our samples. Chlorogenic and cafeic acids increased from RT to TP with the extension of heat treatment. TP2 showed double the content of those acids compared with TP1. On the contrary, ferulic and p-coumaric acids decreased significantly due to the concentration process (Table 2).
The total content of ascorbic acid in the three samples expressed as fresh matter and dry matter is shown in Figure 1. As expected, ascorbic acid decreased significantly from RT to TP1 and from TP1 to TP2. After heating about 30 min at 110ºC, less than 10% of the original ascorbic acid could be found in TP2.
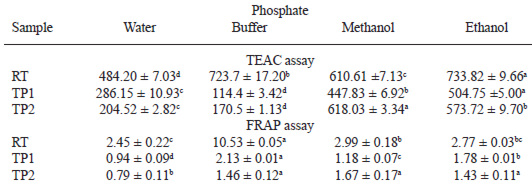
Table 3 shows the antioxidant capacity (TEAC and FRAP assays) in RT, TP1 and TP2, expressed as dry matter, for the different extracts (water, phosphate buffer, methanol and ethanol). The TEAC and FRAP values depend significantly on the extract and the intensity of the thermal treatment applied to the tomato samples. With FRAP as well as with TEAC assays, the antioxidant capacities of both concentrates were significantly lower than those of RT for all types of extracts. A significantly high activity was detected in the RT sample extracted with buffer and assayed using the FRAP method (Table 3). Ascorbic acid content was significantly correlated with AC measured by both methods in water, buffer and methanolic extracts (r>0.9, p<0.01). TEAC of methanolic extracts correlated with TPC (r=0.64, p<0.05) and TF (r=0.92, p<0.01). Related to the individual phenolic compounds, only ferulic and p-coumaric acids showed a positive correlation with the AC of different extracts as measured by TEAC and FRAP. A positive correlation was also observed between the content of quercetin and TF and among cafeic and chlorogenic acids and TPC. But no correlation was detected between the content of lycopene and AC.
We studied the alterations in antioxidant micronutrients contained in tomatoes submitted to moderate heat treatment. We emphasised domestic heating conditions such as those commonly found in households while preparing tomato sauce, etc., not industrial processes that usually include hot break techniques before processing and lower temperatures of thermal treatment. Moderate heat treatment raised the total lycopene content by 30% when samples of tomatoes were heated at 110ºC for 15 min; however the extension of thermal treatment by an additional 15 min led to a decrease of total lycopene in TP2. It is known that homogenisation and heating releases lycopene from its binding sites within the tomato matrix, which allows a better extraction and quantification of the lycopene content in tomato products (12, 16). However, lycopene and other carotenoids can be degraded by oxidative damage when thermal treatment is extended in time and intensity. So the total lycopene content in TP2 decreased moderately, less than 10%, but no isomerization from the (all-E) to (Z)-configuration occurred during thermal treatment. Shi (1) and Zanoni (26) reported that in tomato products cooked at temperatures above 100 ºC, degradation occurred faster than isomerization, increasing as a function of cooking time. For this reason, the losses observed in TP2 could stem from the oxidation of lycopene toward lycopene epoxides, which have not been analysed in this study but could represent more than 5% of the content of lycopene in tomato juices (27). These results agree with those reported by Takeoka (11), who described lycopene concentration decreases of 9–28% after thermal processing of tomatoes into paste, rising with the extension of the heating. So lycopene in tomatoes is relatively resistant to degradation, compared with pure lycopene systems, because other antioxidant constituents, including tocopherol, ascorbic acid and phenolic compounds help to stabilize lycopene during processing (10,11). In addition, the different physical structures of the crystals and membranes associated with lycopene might influence its thermo stability and susceptibility to isomerization. This suggestion is supported by the fact that (all-E)-lycopene isolated from a food matrix isomerised quickly when heated (16), but that did not occur when the lycopene was in tomatoes (10). On the contrary, β-carotene is more labile to thermal treatment, decreasing in total content and increasing its percentage of (Z)-isomers, because β-carotene is susceptible to isomerization, and lycopene is not (5,10,11,18).
Heat treatment caused a significant increase in TPC and TF, mainly due to the breakdown of the cell structure during thermal processing, which allowed a better extraction of those compounds from the food matrix, as described by Gahler (9). Tomato products are considered a good source of flavonol, in which around of 30 % of quercetin is in its free form. In this study, the levels of free quercetin increased slowly in TP2 compared with RT, but the major change was observed in rutin. This flavonol was extracted from tomato skin during processing because the highest amount of conjugated quercetin is accumulated in the skin of tomato fruits (30). These results suggest that processing of tomatoes did not cause a hydrolysis of rutin or other quercetin conjugates, improving their content due to a breakdown of flavonols in the cell wall. In contrast, the content of kaempferol decreased during processing because it is present in a lower concentration in the tomato skin and flesh (30). The flavanone naringenin was not detected in the samples, but some studies have described different levels of naringenin in raw tomatoes and tomato products due to the conversion of naringenin chalcone, present in the skin, into its isomeric flavanone form (31). The other individual phenolic compounds are known to be present in tomatoes in concentrations between 2 and 71 mg/kg (3). Related to the content of phenolic acid, p-coumaric acid was observed to decrease after treatment, probably due to degradation during thermal treatment because these compounds are mainly in the tomato flesh and not in the skin (32,33). On the contrary, hydroxycinnamates (chlorogenic and cafeic acids) increased more than 3-fold from RT to TP2. That increase results from the extraction of these compounds from tomato skins during processing because chlorogenic acid is mainly in this part of tomato fruits (34). Considering that quercetin and chlorogenic acid are considered antioxidants with a beneficial effect for human health, the consumption of processed tomato products might provide a higher amount of dietary phenolic antioxidants than raw tomato.
Results on the behaviour of ascorbic acid were comparable with those in the scientific literature (5,9,12,27). As expected, ascorbic acid was destroyed with the extension of the heat treatment. These results show that domestic cooking might cause greater losses of ascorbic acid than industrial production of tomato paste (28ºBrix), in which vitamin C loss was determined to be about 55% (5). The reason might be that the industrial process was carried out under light vacuum, whereas domestic cooking occurs under normal atmosphere.
The decrease of AC in TP1 and TP2 compare with RT, expressed as dry matter, of the product, is a result of the intensity of thermal treatment, which reduce the relative concentration of antioxidants (9,12). We evaluated both the scavenging capacity (ABTS assay) and the iron-reducing power (FRAP assay) of tomato products. AC was observed in the different fractions, and each extracting solution allowed the extraction of different antioxidant compounds, giving different values. In the TEAC method, the highest values were obtained with methanol and ethanol, with the exception of RT, in which the buffer showed a high scavenging capacity. In FRAP analysis, the highest value was also observed in RT extracted with buffer, showing similar values for the other fractions. This behaviour could be due to a higher extraction of aqueous-phase antioxidants, such as ascorbic acid and glutathione, in the buffer and also to the extraction of antioxidant enzymes that are active in the raw sample (35). We have not investigated the content of glutathione or antioxidant enzymes because we have focused on nutritional compounds, not the physiological mechanisms of the fruit. In this sense, a positive correlation was detected in all fractions between ascorbic acid and AC because vitamin C is one of the antioxidants that contribute to AC in tomatoes (3,29). In addition, other antioxidants, TP, TF and individual phenolic compounds also showed a positive correlation with AC. Taking into account that vitamin C decreased significantly in tomato paste compared with RT, the contribution of phenolic antioxidants seems to be interesting in determining the AC of processed tomato products. However, this effect was observed only in the methanolic fractions and the TEAC assay because the methanolic extract contains mainly phenolic compounds (11). Pulido (36) measured the FRAP values of several polyphenols (quercetin and ferulic and cafeic acids) in water and methanol, and the absorption did not stop at 4 min. Instead, it increased slowly, even after several hours. Therefore, the FRAP cannot provide an accurate value of AC in extracts with phenolic antioxidants. A negative correlation was observed between quercetin, rutin, cafeic and chlorogenic acids because their contents increased in processed products as AC decreased. On the contrary, kaempferol, ferulic and p-coumaric acids showed a positive correlation with the AC of all tomato fractions. The results show that in processed tomato products with a low content of ascorbic acid, AC is determined mainly by the phenolic antioxidants. Because the content of individual phenolic compounds depends on several factors, both agronomical and technological, the measure of total flavonoids and total phenolics can be considered an indicator of AC. No correlation was found between lycopene and other carotenoids and AC because those compounds were not solubilized in the different assayed extracts.
The effects of thermal processing on the nutritional value of tomato paste differ according to the extension of heating. Increases in TPC, TF, individual flavonoids and hydroxycinnamic acids leads to an enhancement of the phenolic antioxidants of tomatoes, which are responsible for maintaining the antioxidant capacity of processed products after losses of ascorbic acid. However, the extension of thermal processing causes a degradation of lycopene, decreasing the contents of both the (E) and (Z) lycopene isomers.
We thank EU VI Frame Programme for the financial support through the IP LYCOCARD-2006-016213 project. The results obtained in this paper only reflect the authors’ view and the Community is not liable for any use that may be made of the information contained herein. The authors are grateful to the Ministry of Education and Science of the Spanish Government for the project AGL 2006-26965-E and to the “Fundación Seneca” of the Murcia Regional Government for the project 05774/PI/07.
Recibido: 25-01-2010
Aceptado: 27-07-2010