The effect of cocoa powder and subchronic exposure to classical music in Wistar rats behavior on anxiety evaluation tests and their antioxidant activity was evaluated. The animals were divided into four groups: control group (CG), cocoa powder group (CPG), music group (MG) and cocoa powder with music group (CPMG). During 15 days, CPG and CPMG received commercial non-alkalized cocoa powder daily (66 mg total polyphenols / g of product, by oral gavage), while MG and CPMG were exposed to the music of Mozart (Serenade N.10 in B flat major for woodwinds and bass, "Gran partita" K.361 / 370a, Largo movement, 8:35 minutes long). At the end of the experiment, the animals were submitted to elevated plus-maze (EPM) and openfield (OF) tests, and serum analysis of thiobarbituric acid reactive substances index (TBA-RS) and the activity of antioxidant enzymes catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px). Animals from MG and CPMG groups showed the highest total horizontal locomotion and more time spent at the central area and reduced immobility time at the OF. The TBA-RS average of the treated groups were lower than the GC. The average activity of CAT was higher in CPMG than the others, and the average activity of SOD and GSH-Px were higher only in CPG and CPMG. We concluded that the treatment with this classical music showed mild anxiolytic activity. Both treatments (cocoa and music) improved serum antioxidant status, but the peripheral activity of different serum enzymes was mainly improved by the cocoa powder.
Key words: Cocoa powder, classical music, anxiety, elevated plus-maze, open-field, oxidative stress.
Se evaluó el efecto de cacao en polvo y la exposición subcrónica a la música clásica sobre el comportamiento de ratas Wistar en pruebas de evaluación de la ansiedad y su actividad antioxidante. Los animales fueron divididos en cuatro grupos: control (GC), cacao en polvo (GCP), música (GM) y cacao en polvo con música (GCPM). Durante 15 días, GCP y GCPM recibieron cacao en polvo comercial no alcalinizado diariamente (66 mg de polifenoles totales / g de producto, mediante una sonda nasogástrica), mientras que GM y GCPM fueron expuestos a la música de Mozart (Serenata N.10 em Si bemol mayor, "Gran Partita" K.361 / 370a, movimiento Largo, 8:35 minutos de duración). Al final del experimento, los animales fueron sometidos a las pruebas de laberinto en cruz elevado (LCE) y de campo abierto (CA), y el análisis sérica del índice de sustancias reactivas al ácido tiobarbitúrico (TBA-RS) y la actividad de las enzimas antioxidantes catalasa (CAT), superóxido dismutasa (SOD) y glutatión peroxidasa (GSH-Px). Los animales GM y GCPM mostraron la mayor locomoción horizontal, más tiempo en la zona central y un tiempo reducido de inmovilidad en el CA. El TBA-RS promedio de los grupos tratados fue más bajo que el control. La actividad media de CAT fue mayor para GCPM que los otros, y la actividad media de la SOD y GSH-Px fueron mayores sólo en GCP y GCPM. Concluimos que el tratamiento con esta música clásica mostró modesta actividad ansiolítica. Ambos tratamientos (cacao y música) mejoraron el estado antioxidante en suero, pero la actividad periférica de diferentes enzimas fue mejorada principalmente por acción del cacao.
Palabras clave: Cacao en polvo, música clásica, ansiedad, laberinto en cruz elevado, campo abierto, estrés oxidativo.
Federal University of Santa Catarina (UFSC), Florianopolis, SC, Brazil. Regional University of Blumenau
(FURB), Blumenau, SC, Brazil. University of Joinville Region (UNIVILLE), Joinville, SC, Brazil.
Anxiety is an unpleasant emotional state, associated to ill-being, discomfort, worry or fear of any defined or undefined future threat. Although it is a natural reaction to stress, when it is excessive or out of proportion to the stimulus, it is considered pathological (1).
The occurrence of mental disorders, among them anxiety, have increased globally along the years. When analyzed as a group, mental and neurological disorders and also those recurring from substance abuse, these accounts for 13% of worldwide illnesses in the year of 2004 and are, today, the fastest growing health related issues. An amount of 16,3 trillion dollars is estimated to be spent on health problems associated to mental disorders between the years of 2011 and 2030 (2). Additionally, aggravating this situation are the side effects of the drugs used to treat anxiety, such as: headache, nausea, blurred vision, tachycardia, dizziness, fainting, hypotension, hypertension, diarrhea, drowsiness, insomnia and vomiting (3).
Faced with these challenges it is both, understandable and necessary, to broaden the range of treatment options or complement them, in areas such as phytotherapy, nutrition and diversified complementary therapies (4). Some foods are of target interest and show good perspectives, but in order for them to be indicated for use, scientific comprobation is necessary.
Cocoa powder, a sub product of the Theobroma cacao L. fruit, shows high levels of bioactive compounds, taking into consideration that it has a larger proportion of cocoa solids. When it is not alkalized during processing it shows significant amounts of polyphenols like flavonoids, epicatechins, catechins, proanthocyanidins and quercetin derivatives, and methylxanthines like theobromine and caffeine (5). Due to its composition, it offers many applications, such as: antioxidant, antiinflammatory, antithrombogenic, fibrinolytic, immunomodulatory and antitumoral (6,7). Although still poorly explored, recent evidence has been found of the antidepressant and anxiolytic effects of cocoa. These were found in pre-clinical studies through behavioral testing on animals (8-10) and more modestly on humans trials (11). These effects were associated to cocoa's complex composition, but mostly due polyphenols.
Music is a complementary therapy that has been used by humanity for thousands of years and scientific studies which indicate music as a therapeutic method for many conditions have emerged (4). Many studies describe that music causes metabolic alterations to the regulation of the hypothalamic-pituitary-adrenal axis (HPA), the sympathetic nervous system and the immune system, besides reducing anxiety (12,13). Classical music, particularly by Mozart, has demonstrated effects on cortisol levels (14,15), modulation of the HPA axis as well as the immune system (16) in humans. Experimental studies with the exposure to music from this particular composer showed reduction of hypertension (17) and anxiety isolatedly in rodents (18) and also when combined with medication (19).
Both, human and animal organisms, respond to physical and psychological stress through behavioral and physiological defenses (13). Growing evidences indicate that oxidative stress plays causal role in the development of anxious behavior on rats (20-23) and due to the fact that cocoa has conferred antioxidant effects and as well as classical music it may work as a potential anxiolytic, this study sought to verify the effects of an adjoint intervention of these two factors on Wistar rats behavior when submitted to the elevated plus-maze (EPM) and also to the open-field (OF) tests, anxiety evaluation models, associating it to the peripheral antioxidant defense.
A total of 24 male genetically heterogeneous albino Wistar rats (Rattus norvegicus), 3 to 5 months and weighing 225 g on average (SD = 9 g), were obtained from the animal house of the Regional University of Blumenau. After arrival in the vivarium of the laboratory, these animals were housed individually in opaque plastic cage (0.5 x 0.3 x 0.15 m) with wood shaving bedding and wire mesh tops. They were housed under a standard (12 h/12 h light/dark; cycle lights on at 7:00 AM), in a temperature-controlled environment (23 ± 1ºC), with a 50 dB background sound level, and 55±10% relative humidity. During the entire experimental period, the animals received standard commercial chow for rodents (Nuvital®) and filtered tap water ad libitum. The room was visited on an average of once every 2 or 3 days for cleaning cages, and to provide food and water. The animals were acclimated to the animal housing facilities for 1 week before the experiments began. This study was approved by the university Ethics Committee for Animal Research (CEUA) by the protocol No. 125/14. The experiments were performed in compliance with the recommendations of the Brazilian Society of Neuroscience and Behavior (SBNeC), which are based on the United States National Institutes of Health Guide for Care and Use of Laboratory Animals.
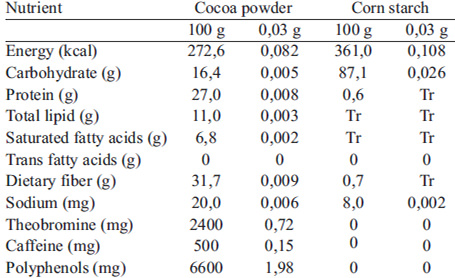
The animals were divided into four groups: control group (CG), cocoa powder group (CPG), music group (MG) and cocoa powder with music group (CPMG); each comprising six animals. During the intervention period, which lasted 15 days, the animals of CPG and CPMG groups received once a day, between 12:00 and 1:00 PM daily, non-alkalized cocoa powder solubilized in water (by oral gavage). The commercial nonalkalized cocoa powder (natural) (66 mg total polyphenols / g of product) was purchased from Brazilcoa ® and has its origins in the brazilian states of Bahia, Espírito Santo and Rondônia. Each animal received 2.5 mL of solution containing 134 mg of cocoa powder / kg of animal weight, diluted in water at a ratio of 0.03 g: 2.47 mL (cocoa powder : water). The solution was daily prepared immediately prior to administration and with the aid of a magnetic stirrer at 625 rpm (Quimis® model Q261-2) and a precision balance (Marte Científica® model AS1000). The solution contains about 8.86 mg total polyphenols / kg body weight, the minimal dose at which changes were observed in behavioral tests (8). The CG and MG animal groups received 2.5 mL of placebo (by oral gavage) containing corn starch in replacement of the cocoa powder. The chemical composition of both solutions is presented in Table 1. Corn starch was used as placebo to ensure that the infusion time (gavage) was the same for control and treatment groups, giving density to the solution. This was an important consideration as the gavage procedure can generate cause stress to the animals.
The rats of MG and CPMG groups were exposed to classical music for 5 hours / day between 1:00 and 6:00 PM, during the 15 days of trial. Mozart's music (Serenade N.10 in B flat major for woodwinds and bass, "Gran partita" K.361 / 370a, Largo movement, 8:35 minutes long) was continuous and repeated in a CD player (compact disc) (19). The speaker had a frequency range of 100-16000 Hz and the music room had a sound level of 65-75 dB. The silent room where the animals of CG and CPG groups stayed had no music and its sound level was 50 dB (ambient noise).
The animals body weight was measured on the first and last day of the intervention and food consumption was measured three times a week on alternate days and then, the weekly average was calculated. In both procedures, a precision scale was used (Marte Científica® model AS1000).
On the last day of intervention all animals were individually subjected to the EPM and OF tests in a sound-isolated room, during the light phase of the cycle (between 1:00 and 4:00 PM). To minimize possible circadian influences, the experimental and control observations were alternated. The observer stayed in the same room approximately one meter away from each apparatus and recorded the tests with a video camera (Panasonic® PV-GS150 model) for later behavioral analysis (24,25). The tests were conducted under dim red light (44 lux).
The apparatus consisted of two open arms (0.5 x 0.1 m) and two closed arms (0.5 x 0.1 x 0.4 m) arranged such that the two arms of each type were opposite to each other, with a central platform (0.1 x 0.1 m). The height of the maze was 0.5 m. The animals were exposed for 5 min to the red light in their own home cages before the testing procedure. They were then individually placed on the central platform of the elevated plus-maze facing an open arm. During a 5 min test period the animal behavior was recorded and the following parameters were registered: time spent in open arms and the percentage of time spent in the open arms relative to the total time in the arms; number of entries into the open arms; percentage of open arm entries compared to total entries; time spent in the closed arms; number of entries into the closed arms and risk assessment (25). Risk assessment is a measure that accounts for the time spent head-dipping (i.e., exploratory movements of the head/and shoulders over the side of the maze) and in a stretched attend posture (i.e., exploratory posture in which the body is stretched forward and then retracted to the original position without any forward locomotion). The measures that reflect anxiety-like behavior in this test are the entries into the open arms vs. closed arms and time spent on the open arms vs. closed arms. The anxiety behavior in this test is triggered by the high of the maze which evoke a greater strength of fear. The fear induces an avoidance behavior, which in turn makes the animals prefer the closed arms (25). We also included ethologically derived measures related to the defensive pattern of risk assessment behavior, which has been shown to be very sensitive to changes in anxiety (19, 26).
The OF consisted of a black uncovered circular box (0.6 m diameter, 0.50 m height). Each rat was placed in the central area and allowed to freely explore the apparatus for 5 min, being filmed during this time. Subsequently we recorded the total ambulation time (i.e., movement from one location to another); total horizontal locomotion, estimated by the number of squares crossed (every time both hind paws entered one square, a crossing was recorded); peripheral area ambulation time; peripheral area crossings; central area ambulation time; percentage of time spent in central area compared to total ambulation time; crossings made to the center of the field and percentage of crossings made to the center of the field in relation to total horizontal locomotion. Time spent immobile (i.e., completely immobile), time spent rearing (the rat stood on its hind paws with its body at greater than a 45° angle to the floor) and time spent grooming (i.e., repetitive movements of the front paws or mouth on the fur) were also recorded. The measures that reflect anxiety-like behavior in this test are shorter horizontal locomotion (number of crossings); greater time spent in the peripheral area; reduced ambulation time; reduced central area ambulation time; and greater immobility time. The anxiety behavior in this test is triggered by the fact that it is an individual testing (the animal is apart from its group) and the area is very large in relation to the animal’s breeding or natural environment, which is why they prefer the peripheral area, close to the wall. Anxiolytic treatments decrease the stress-induced inhibition of exploratory behavior (24).
At the end of the experiments the animals were sacrificed by decapitation with guillotine (Insight® model EB271). Erythrocytes and plasma were prepared from whole blood samples obtained from the rats. Whole blood was collected and transferred to heparinized tubes for erythrocyte separation. Blood samples were centrifuged at 1,000×g (Luguimac® model LC-10), and plasma was removed by aspiration and frozen at – 80 °C until determination of thiobarbituric acid reactive substances (TBA-RS). Erythrocytes were washed three times with cold saline solution (0.153 mol/L sodium chloride). Lysates were prepared by the addition of 1 mL of distilled water to 100 μL of washed erythrocytes and frozen at – 80 °C until determination of the antioxidant enzyme activities. For antioxidant enzyme activity determination, erythrocytes were frozen and thawed three times and centrifuged at 13,500×g for 10 minutes. The supernatant was diluted in order to contain approximately 0.5 mg / mL of protein. TBA-RS was measured following Ohkawa et al. method (27) and was determined by the absorbance in spectrophotometer (Metrolab® model 325-DB) at 535 nm. The results were expressed as nanomole of malondialdehyde formed per milligram of protein. Catalase activity assay (CAT) was determined according Aebi method (28). Hydrogen peroxide (H2O2) disappearance was continuously monitored with a spectrophotometer at 240 nm for 90 seconds. One unit of the enzyme is defined as 1 μmol of H2O2 consumed per minute, and the specific activity is reported as units (U) per mg of protein. Glutathione peroxidase activity assay (GSH-Px) was determined by the method of Wendel (29), with some modifications. Tert-butylhydroperoxide was used as substrate. Nicotinamide adenine dinucleotide phosphate (NADPH) disappearance was continuously monitored with a spectrophotometer at 340 nm for 4 minutes. One GSH-Px unit is defined as 1 μmol of NADPH consumed per minute, and specific activity is reported as units per mg of protein. Superoxide dismutase assay (SOD) was determined by the pyrogallol auto-oxidation method, as described by Marklund (30). The self-oxidation of pyrogallol was continuously monitored with a spectrophotometer at 420 nm. The specific activity was expressed as unit per mg of protein. Protein determination was performed by the method of Lowry et al. (31), using bovine serum albumin as the standard.
The variables were expressed as means and standard deviations. The determination of the differences between the experimental groups was performed using analysis of variance (ANOVA), two-tailed (food intake and body weight) and one-tailed (other variables), with Tukey-Kramer post-test (for parametric data) or Kruskal-Wallis test (for non-parametric data). The Normality of variable distribution was assessed by Kolmogorov-Smirnov test. All tests were performed with the software Statistic (StatSoft Inc®, version 6.0). Differences with p <0.05 were considered significant.
No significant alterations were observed in body weight, percentage of gained weight and weekly food intake. The food intake of the first and second weeks of trial did not differ from each other (data not shown).
No statistical differences were observed between groups in the parameters analyzed in the EPM test.
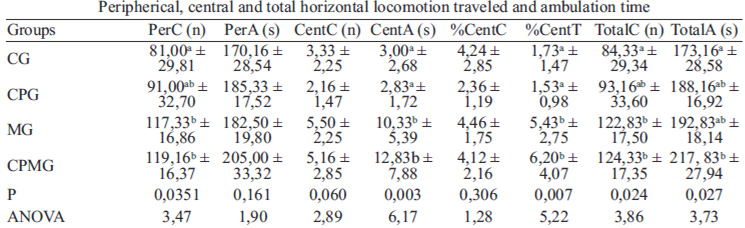
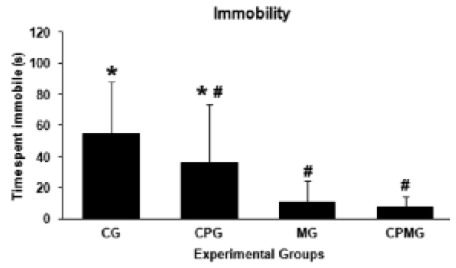
The ANOVA revealed significant differences for some of the OF test variables (table 2). A larger number of peripheral crossings was verified for the CPMG and MG groups when compared to CG (p=0,035), even though the ambulation time in the peripheral area did not differ among the groups (p=0,161). Likewise, animals of CPMG and MG groups spent more time ambulating in the central area of the field than CPG and CG (p=0,003). The animals exposed to music and also those with the combination of music and cocoa showed higher percentage of time ambulating in the central area regarding the total ambulation time in relation to CPG and CG (p=0,007). Nevertheless, the number of crossings made to the central area did not differ between the animals (p=0,061). The total horizontal locomotion (total number of crossings) performed by the CPMG and MG groups was greater than CG (p=0,025), and the average of this locomotion measure traveled by CPG was statistically the same as CG. The CPMG showed a total ambulation time higher than the GC group while the CPG and MG groups showed average values equal to control (p=0,028). Considering the ethological measures analyzed, a significant decrease of immobility time was found in CPMG and MG groups when compared to the CG (F = 4,35; p=0,016) (Figure 1).
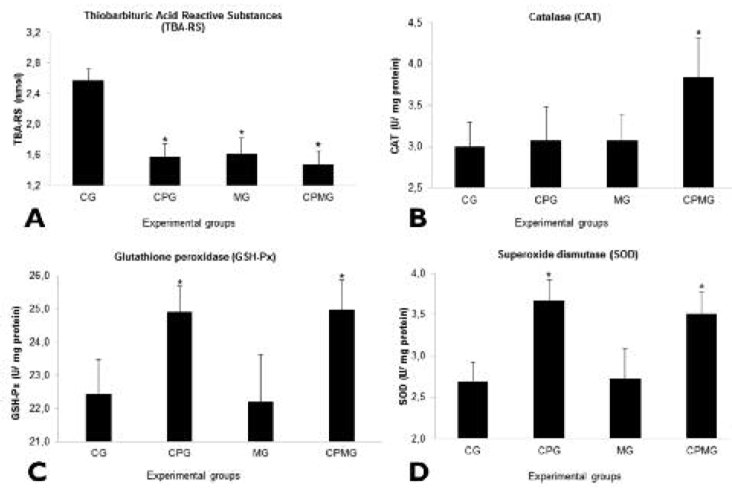
Serum concentrations of antioxidant markers and oxidative damage analysis at the end of the experiment (Figure 2) revealed that averages of TBA-RS were inferior for the animals of CPMG, CPG and MG groups (F = 50,45; p<0,001) (Figure 2A). As to enzymatic activity, the average activity of CAT was statistically superior for the CPMG group when compared to all the other groups (F= 6,73; p=0,002) (Figure 2B). The averages of GSH-Px (F= 11,95; p<0,001) (Figure 2C) and SOD (F= 19,82; p<0,001) (Figure 2D) were superior for the CPMG and CPG groups when compared to the control.
In this study, the cocoa treatment associated or not to classical music did not cause alterations in body weight gain or animal food intake. Likewise, other authors did not verify changes to these variables when the same dose of polyphenols used in this study were administered (8,86 mg/kg) through cocoa polyphenolic extract (8). The cocoa mass treatment (250 mg/kg weight/day) also did not show alterations in body weight (10).
Regarding the EPM test, this study differs from one of our previous works using Mozart music in combination with medication (simvastatin), in which we observed an adjuvant effect of the interventions in reducing anxiety (19). A recent study published in pertinent literature may explain such differences in these results. According to Attoui et al. (18), when the animals were exposed to auditory stress (105 dB) and/or to predator odor for 10 minutes/day, during 7 days, associated to Mozart’s music 10 minutes before the EPM test, there was a reversion in the anxiety behavior and also a reduction in the levels of the adrenocorticotropic hormone (ACTH) of the stressed rats, independent to the kind of stress to which they were submitted. It is believed that as the animals in this present study were not exposed to stress stimuli, there was no hormonal deregulation to be reverted by the treatment with music, if we consider this the mechanism of action. However, it is relevant to emphasize that there were significant differences in the OF test variables; an anxiety behavior model with different peculiarities regarding action mechanisms. Therefore, the necessity of applying not only one paradigm to evaluate stress or emotional anxiety to behavior studies is reinforced. During OF testing a new environment is used to determine general anxiolytic behavior and mobility levels in this paradigm serve as figures for a similar state of anxiety in rats, possessing sensitivity to serotonergic and benzodiazepine routes of action (acting on the inhibitory neurotransmitter GABA - Gamma-Aminobutyric acid) (24).
Either as a separate treatment or associated with cocoa powder, the classical music treatment promoted an increase in the total horizontal locomotion in the OF and also the horizontal peripheral locomotion, but did not affect the time spent in this region. It is also important to highlight that both treatments significantly increased the time spent in the central area of the field, and also the percentage of time spent in this area when compared to total ambulation time, which represents a rupture in the natural tendency of the rodents of moving to the peripheral area (24). In agreement to these results, it was found that the CPMG and MG groups had significant reduction in immobility time, when compared to CG.
Taking into account the results seen in the OF test and that we saw no differences in the animals behavior at the EPM, it is possible to indicate a mild anxiolytic effect associated with an exploratory activity stimulation, because as said before, anxiolytic treatments decrease the stress-induced inhibition of exploratory behavior at the OF (24). It is believed that this effect may be attributed mainly to classical music exposition, considering the results from CPMG and MG groups were similar, with exception to the total ambulation time (higher than the control group only in the associated treatment of cocoa powder and classical music). Similar results were found in a study in which classical music in combination with simvastatin increased the ambulation time and total horizontal locomotion, also reducing the animal’s immobility time (19).
There isn't a consensus regarding the appreciation of music by animals, however this determination is not necessary, taking into account that many mechanisms used to test its effect on anxiety and stress have already been reported in the literature. Mozart's music increases dopamine (excitatory neurotransmitter) synthesis in the brain. This is due to its high frequency accentuated sounds (4000 a 16000 Hz) compared to other classical music composers, endowing rats with greater neurophysiologic effects, confirmed through alterations in autonomic responses such as blood pressure (17). Furthermore, music also significantly activates various subcortical regions in humans, including the nucleus accumbens (NAc, cerebral area that plays a significant role in processing of motivation, pleasure, reward and reinforcement), the ventral tegmental area (VTA, processes reward when it receives dopamine) and the hypothalamus (region of the brain that modulates autonomous responses such as cardiac frequency and breathing, which alter when exposed to pleasant musical sounds). The elevated dopamine release in the VTA and NAc may be the neurochemical mechanism which can explain the reward feelings brought by music (16, 32). The stimulus of structures such as the amygdala, cortex, hippocampus and hypothalamus by music and the way the brain's attention channels are affected by soothing auditory stimuli in a significant and distractive manner are also mentioned explanations (13).
In addition to the previous consideration, a musical approach has shown regulatory effects on the HPA axis, by reducing the levels of ACTH in stressed rats (18), reducing salivary cortisol levels in humans under precompetition stress (15), as well as in the absence of stress (14). Stress shows consequences such as high blood pressure and endocrinal responses which lead to specific hormone release and also the activation of the sympathetic nervous system for the "fight or flight" response, besides an immune response, showing alteration in markers such as Immunoglobulin-A, interleukins (IL) 1,6 and 10 (13). The "fight or flight" response may be triggered by mental activity such as anxiety, stress, depression and feelings of despair. As previously discussed, music reduces stressful and anxious behavior (13) and it eases some of these systemic markings (not only the ACTH and cortisol), with the reduction of epinephrine and IL-6 and also the elevation of the growth hormone (GH) in humans. It appears that this effect of music concerns neuroendocrine-immune routes of action (12,16).
In this study, besides the fact that the cocoa powder treatment did not provoke a statistically significant modification in animal behavior, it is important to note that, even not significantly different of control, it increased the average of peripheral crossings as well as the total ambulation time reducing the animal's time of immobility. It is noteworthy that only the group which received cocoa powder in association with music demonstrated a greater total ambulation time compared to the control group. It is possible that there may have been an adjuvant effect between the treatments, which did not manifest statistically when they were both conducted in an isolated manner. Pertaining the usage of the cocoa powder, aspects such as the bioavailability of the polyphenolic compounds, compromised by the food matrix, interaction with diet constituents, genetic factors, microbiota metabolism and the enzymatic activity of the colon (7) should be taken into account, since the intake of the same dosage of total cocoa polyphenols caused behavioral alterations to rats in a previous study (8).
The use of cocoa in anxiety reduction is supported by the literature in various courses of action. Because its bioactive compounds are elevated, it is believed that there may be a synergetic action between the components in the production of the anxiolytic effect (10). Cocoa polyphenols have been associated to elevation in the monoaminergic neurotransmitters, similarly to antidepressant drugs. As evidenced in the work of Yamada et al. (10) when chronically administered (14 days), the cocoa mass (100 mg/ kg weight) led to a tendency, not significant, of increase in the total distance traveled in the OF (similar to the results in this study), a behavior which was associated to greater concentration of serotonin in the cortex, hippocampus and amygdala. Messaoudi et al. (8) verified a smaller time of immobility of the animals submitted to forced swimming test when using cocoa polyphenol extract (24 mg/kg weight) for 14 days, however, no changes in the OF test. Such behavioral effect has been suggested to the quercetin (flavonoid), for acting in a similar manner to selective serotonin reuptake inhibitors drugs such as tricyclic antidepressant.
Considering the existence of a direct correlation between oxidative stress and anxiety, the brain's high susceptibility to oxidative stress and the already proven antioxidant efficiency of cocoa, it is possible that this may also be a way of reducing anxiety. When Sabogal-Guaquetá et al. (9) administered quercetin (flavonoid present in cocoa) to rats (25 mg / kg), they were able to reverse the main histopathological markers of cognitive and emotional dysfunction, besides demonstrating anxious behavior reduction in the EPM test.
Evidences that oxidative stress plays a causal role in the development of anxious behavior in rats has increased, as well as the evidences that an antioxidant intervention may revert such effects (23). The gene expression of antioxidant enzymes is different for anxious and non-anxious rats in specific cerebral regions (20), just as oxidative stress provokes an imbalance to antioxidant enzyme activity in the hippocampus associated to the anxious behavior in rats during the OF test (21). The same imbalance to the antioxidant defense was verified beyond the CNS, in many psychiatric disorders (among which, anxiety) (22). In spite of the fact that cocoa has an already established role in antioxidant defense improvement, the same does not apply to classical music, once no work in the literature has directly evaluated peripheral antioxidant activity with this treatment, within our knowledge.
In this study, all interventions, either associated or isolated, provoked a reduction to TBA-RS levels in serum when compared to the control group (Figure 2A). The malondialdehyde, a secondary product of lipid peroxidation, reacts to the TBA and is measured colorimetrically through the TBA-RS test (27). It was found that the cocoa powder, Mozart’s music and its association reduced plasmatic lipid damage. Cocoa powder itself contribute to a reduction in LDL cholesterol and the suppression of oxidized LDL in humans (6). The efficiency with which the polyphenols are incorporated to LDL surface, thus increasing the resistance of this particle to both oxygen radicals and chelating transitional metal ions, is an exogenous antioxidant mechanism suggested for such compounds (6).
Furthermore, endogenous antioxidant activity was also altered by treatments with cocoa powder and classical music, verified through the increase of enzymatic activity in serum. The average activity of the CAT enzyme was statistically superior only for the group treated with cocoa powder and concurrently exposed to music (Figure 2B). The average activity of the GSHPx (Figure 2C) and the SOD (Figure 2D) enzymes were superior for the groups CPMG and CPG when compared to the others, which demonstrates an inherent effect of the cocoa powder. A cocoa powder enriched diet elevated SOD and CAT activity in young rats in a work of Ramiro-Puig et al. (33). Considering that the increase in CAT activity in our study was exclusive to the CPMG group, the existence of a common or synergetic mechanism between the interventions (cocoa and classical music) is probable but still unexplored by science. In addition to this, the treatment with music reduced lipid peroxidation of the animals, probably through indirect mechanisms. A possible pathway is the modulation of the HPA axis activity induced by Mozart’s music, because this has multiple systemic effects on stress markers (13) and the balance between oxidation and antioxidation is affected by signs of the neuroendocrine stress response system (34).
In this study, we concluded that the cocoa powder, in the adopted dosage and used isolated, did not cause significant changes to animal behavior. Nevertheless, it did infer a tendency to a mild anxiolysis effect when combined to Mozart’s classical music. The treatment with classical music by itself did demonstrate a mild anxiolytic effect. Both treatments (cocoa powder and classical music) improved the serum antioxidant status. In view of the above, it is believed that the constituents of cocoa powder (specially the polyphenols) and Mozart’s classical music may have acted together on the improvement of the antioxidant response and, possibly through this, on the anxious behavior of rats. This field of work needs further investigation in order to investigate the correlation between oxidative stress and anxiety. A larger dose of cocoa is recommended in order to consider the bioavailability of the polyphenolic compounds in the food matrix. More behavioral tests and also verification of antioxidant activity in structures of the central nervous system are recommended.
Recibido: 07-11-2016
Aceptado: 17-01-2017