Naranjilla (Solanum quitoense Lam.) is a native fruit of the Andes, cultivated and consumed mainly in Ecuador, Colombia, and Central America. Because of its pleasant aroma and attractive color, it has high potential as an ingredient of products such as juices, nectars, and jams. The main characteristics of mature naranjilla fruits cultivated in Costa Rica were assessed, including sugar content, total titratable acidity, total soluble solids, oxygen radical absorbance capacity (H-ORAC), and total polyphenolic and ascorbic acid content. Carotenoid and volatile compound identification was also done. The samples showed sucrose, glucose, and fructose content of 1.6 ± 0.3, 0.68 ± 0.05, and 0.7 ± 0.1 g/100 g, respectively. Total titratable acidity was 2.63 ± 0.07 g citric acid equivalent / 100 g and total soluble solids amounted to 9.1 ± 0.5 ºBrix. H-ORAC value was 17 ± 1 μmol Trolox equivalent / g, total polyphenolic content was 48 ± 3 mg gallic acid equivalent / 100 g and ascorbic acid content was 12.5 ± 0.0 mg/100 g. Carotenoid content of the whole fruit and pulp was 33.3 ± 0.6 and 7.2 ± 0.3 μg/g, respectively. The predominant carotenoid among the compounds identified in the whole fruit was ß-carotene. Ten volatile compounds were identified in naranjilla pulp, the predominant being methyl butanoate. The chemical composition of naranjilla cultivated in Costa Rica does not seem to differ from that previously reported in studies at different locations.
Key words: Solanum quitoense Lam., chemical composition, antioxidants, volatile compounds.
La naranjilla (Solanum quitoense Lam.) es una fruta nativa de los Andes, cultivada y consumida principalmente en Ecuador, Colombia y América Central. Las principales características de frutas de naranjilla maduras cultivadas en Costa Rica fueron evaluadas, incluyendo contenido de azúcares, acidez titulable total, sólidos solubles totales, capacidad de absorbancia de radicales de oxígeno (H-ORAC) y contenido de polifenoles totales y ácido ascórbico. La identificación de carotenoides y compuestos volátiles fue también realizada. Las muestras presentaron contenidos de sacarosa, glucosa y fructosa de 1.6 ± 0.3, 0.68 ± 0.05 y 0.7 ± 0.1 g/100 g, respectivamente. La acidez titulable total fue 2.63 ± 0.07 g equivalentes de ácido cítrico / 100 g y los sólidos solubles totales fueron 9.1 ± 0.5 ºBrix. El valor de H-ORAC fue 17 ± 1 μmol equivalentes de Trolox / g, el contenido de polifenoles totales fue 48 ± 3 mg equivalentes de ácido gálico / 100 g y el contenido de ácido ascórbico fue 12.5 ± 0.0 mg/100 g. El contenido de carotenoides de la fruta completa y la pulpa fue 33.3 ± 0.6 y 7.2 ± 0.3 μg/g, respectivamente. El carotenoide predominante en los compuestos identificados en las frutas completas fue ß-caroteno. Diez compuestos volátiles fueron identificados en la pulpa de naranjilla, siendo el predominante el butanoato de metilo. La composición química de naranjilla cultivada en Costa Rica aparenta no diferir de aquella reportada previamente en estudios realizados en lugares diferentes.
Palabras clave: Solanum quitoense Lam., composición química, antioxidantes, compuestos volátiles.
Centro Nacional de Ciencia y Tecnología de Alimentos (CITA), Universidad de Costa Rica, San José, Costa Rica.
Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD),
Département Performances des systèmes de production et de transformation tropicaux (PERSYST), France
Naranjilla (Solanum quitoense Lam.) is a native fruit of the Andes, cultivated and consumed mainly in Ecuador, Colombia, and Central America. The tree grows best between 1200 and 2300 m above sea level (m asl); it is neither a tropical nor a temperate plant (1). The fruit is round and its peel is covered with brittle hairs. During ripening, naranjilla turns from green to orange, and the fruit is climacteric, with a relatively low respiration rate even during the climacteric peak (2). Even though the fruit is sometimes consumed and processed at earlier stages of maturity (due to the pulp’s attractive greenish color), total soluble solids increase with maturity, which results in a sweeter pulp at later stages. The pH does not vary significantly with maturation (3).
The fruit contains four compartments separated by membranous partitions and filled with pulp, which has a somewhat acidic flavor and a pleasant delicate aroma. It has numerous flat and hard seeds. The flavor of naranjilla has been described as sweet, similar to a mixture of banana, pineapple, and strawberry (4).
According to Heiser and Anderson (1), naranjilla trees were introduced over a half century ago to Costa Rica. Current estimates indicate about 30-50 ha cultivated with the crop. Fruits are usually sold fresh in local markets and supermarkets and are used to produce beverages, with the addition of water and sugar. As for processed products, naranjilla jam may be the only currently produced in commercial quantity in Costa Rica.
Despite the fact that naranjilla pulp is mostly consumed in Latin America, either in fresh form or as a sweetened drink, authors agree that the fruit has high potential as an ingredient for products such as juices, nectars, ice creams, candies, jams, jellies, toppings, sherbets, sauces, and other cooked confections (5, 6). The pulp’s green color may be an advantage for the food industry, but it should be protected from oxidation so it does not turn brown that quickly.
Since ripe naranjilla fruits are highly susceptible to mechanical damage and fungal attack, fruits are usually harvested green, but when they reach physical maturity. Therefore, fruits are more easily managed and marketed for a longer time. It should be considered that fruits harvested at later maturity stages show better quality characteristics when ripe that those harvested at earlier stages (3). At ambient temperature, the shelf life of physiologically mature fruits is only 6-8 d long after harvest (2, 5). Arango et al (2) recommended several postharvest handling systems for naranjilla and concluded that fruits at physiological maturity can be maintained green for 50 d when packed inside polyethylene bags with an ethylene absorber and refrigerated at 7.5ºC. Morton (5) indicated that fruits picked half-colored can be stored for 1 or 2 mo at 7.22-10ºC and relative humidity of 70-80%.
As for volatile constituents of naranjilla, Brunke et al (4) used gas chromatography / mass spectrometry analysis and sensory evaluation with a gas chromatographic sniffing detector to identify components and determine their influence on the flavor of the fruit. It was determined that the methyl and ethyl esters of lower carboxylic acids contributed much to the typical naranjilla flavor, but it was not possible to find one or more impact compounds, which made researchers suspect that the yet unidentified substances that possess strong odors play an important role in the flavor complex of the fruit. Osorio et al (7) concluded that glycosides isolated from Solanum quitoense leaves have a role as flavor precursors because they produce several volatile compounds with pleasant sensorial notes by the action of some enzymes present in the fruit. These generated volatile compounds may be important with respect not only to the fruit flavor but also to the flavor of the processed products because they would be released during processing. In a different study, with the aid of multilayer coil countercurrent chromatography, subsequent acetylation, and liquid chromatographic purification of a glycosidic mixture obtained from S. quitoense leaves, three C13-norisoprenoid glucoconjugates were isolated by Osorio et al (8) in pure form.
There is great similarity between S. quitoense L. and S. vestissimum D. with regard to both the shape of the plant and the aspect of the fruit. However, aromas are rather different. Authors have extensively studied the volatile constituents of the second fruit (9), glycosidically bound aroma compounds from its pulp and peelings (10), change in volatile compounds during maturation (11), and volatile constituents from its peelings (12).
The characterization of naranjilla cultivated in Costa Rica, in terms of chemical composition, antioxidant properties, and volatile constituents will be critical in increasing this crop’s value in local and regional markets, as a fresh fruit and as raw material for processed products.
Fully mature naranjilla fruits were harvested from several trees in a plantation located in Heredia, Costa Rica (Helénica Proverde S.A.). The agroecological characteristics are as follows: geographical position 10º 01' 59'’ N, 84º 02' 41'’ E; soil of volcanic origin; altitude, 1360 m asl; annual average precipitation, 2479.7 mm; annual average temperature, 18.7ºC; and annual average relative moisture, 87%. Fruits were manually sorted for physical and microbiological damage and then cleaned, washed, and sanitized by immersion for 3 min in a 150-ppm solution of sodium hypochlorite. Only ripe fruits (75-100% orange color in peel) were analyzed.
Analyses were conducted on four separate fruit lots (obtained from the same location but on different dates). Fruits were cut in halves and pulp was extracted by using an industrial finisher with a 1.5-mm sieve. After the pulp was obtained, one part was taken for moisture, total titratable acidity, pH, total soluble solid, and color analyses. The rest of the pulp was immediately frozen by immersion in liquid nitrogen, and a portion was used to determine total polyphenolic content. The remainder was then freeze-dried and stored at -20ºC in laminated bags until further analyses. For carotenoid and volatile compound analyses, whole fruits from two of the four lots were frozen until analyses.
Moisture, ash, protein, dietary fiber, total titratable acidity (expressed as citric acid equivalent) and pH were determined using standard AOAC methods (13). Fat content was determined using the Soxhlet method (ether extraction) (14). Available carbohydrate content was determined by difference, from moisture, ash, protein, fat, dietary fiber, and total titratable acidity analyses. Total sugars (sucrose, glucose, and fructose) were determined using a Shimadzu LC-6A highperformance liquid chromatography (HPLC) system, equipped with an Alltech Econosphere NH2 column with a mixture of acetonitrile and bidistilled water for the mobile phase. Energy content was calculated using the general factors of 4, 4, and 9 calories per g of protein, carbohydrate, and fat, respectively (15). Color was measured with a HunterLab ColorFlex CX1192 colorimeter.
Antioxidant capacity was measured in terms of hydrophilic oxygen radical absorbance capacity (H-ORAC), following the method described by Ou et al (16) and adapted for manual determination by Vaillant et al (17), expressing results as μmol Trolox equivalent / g. Total polyphenolic compounds were determined by using a modified Folin-Ciocalteu assay described by Georgé et al (18) with gallic acid as standard and expressing the results as mg gallic acid equivalent / 100 g, after corrections for interfering substances. By the same methodology, ascorbic acid content was determined (using an ascorbic acid standard) and results were expressed in terms of mg ascorbic acid/100 g (18).
For total carotenoid analyses, frozen fruits were thawed and contents were assessed on the whole fruit (pulp and peel), pulp, and seedless juice. Homogenized samples were mixed with acetone for extraction of carotenoids. The liquid was then filtered and concentrated, and the carotenoids were extracted with ethylic ether/n-hexane (50/50, v/v). The organic phase was dried under a nitrogen flow, and the remnant was dissolved in ethylic ether and saponified by mixing with potassium hydroxide. The organic phase was separated and the aqueous phase was extracted again with ethylic ether/n-hexane (50/50, v/v). The total organic phase was mixed with a sodium chloride solution to eliminate alkali, and then separated and dried under a nitrogen flow. A precise amount of n-hexane was added to a portion of the remnant and absorbance was read at 450 nm with a UV-1700 Shimadzu UVvisible spectrophotometer. Total content of carotenoids was estimated by using the extinction coefficient of ß-carotene at a 1% concentration in n-hexane (2500). The same sample was used for identification of carotenoids, using a Hewlett Packard 1050 reverse-phase HPLC system coupled to a diode array detector. The HPLC was equipped with a Spherisorb ODS-2 HP (250 mm × 4 mm × 5 μm) column. The mobile phase was a mixture of acetonitrile, dichloromethane, and methanol (82:13:5); flow rate was set at 1.5 mL / min; and the determination was performed at 20ºC. For identification of carotenoids, only whole fruits were analyzed.
To identify the volatile compounds, frozen whole fruits were thawed and aroma compounds were assessed on the seedless pulp. Extracts were obtained by mixing 2 g of pulp with 30 mL of pentane/ether (50/50, v/v) and then homogenizing using a Potter Elvejhem homogenizer for 5 min at room temperature. The organic phase was recovered, dried with anhydrous sodium sulfate, and concentrated at 37ºC to 1 mL with a 25-cm Vigreux distillation column (19). GC-MS analysis was used to identify volatile components using a Hewlett-Packard 6890 gas chromatograph coupled to a Hewlett-Packard 5973 quadrupole mass spectrometer. The column was a DB-WAX fused silica capillary column (30 m, 0.32 mm i.d., 0.25 μm film thickness), preceded by a 2 m, 0.32 mm i.d. uncoated pre-column. Oven temperature was set at 40ºC for 3 min, then increased to 245ºC at 3ºC/min, and kept at this temperature for 20 min. The on-column injector was heated from 20ºC to 245ºC at 180ºC/min. The detector and injector temperatures were 250ºC. Helium was the carrier gas at 1.1 mL / min. The electron impact energy was 70 eV and the ion source and quadrupole temperatures were 230ºC and 150ºC respectively. Electron impact (EI) mass spectra were recorded in the 40-600 amu range at 1-s intervals. Injection volume was 2 μL of extract. Volatile compounds were identified by comparing their spectra with those of the Wiley 275 and NIST MS libraries.
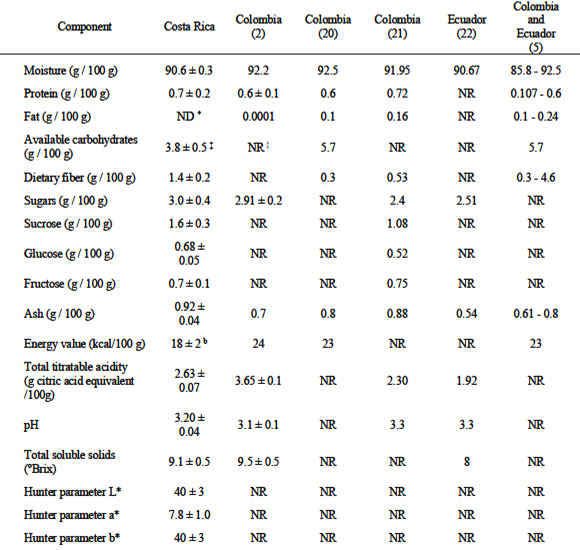
The chemical characterization of Costa Rican naranjilla pulp, as well as results found in literature for ripe fruits from Colombia and Ecuador are summarized in Table 1. When comparing results, factors such as sampling, plotting, sample preparation, extraction, and measurement, besides genetic and environmental factors (maturity, UV light exposure, etc.) should be considered. Total antioxidant capacity of naranjilla pulp was measured in terms of H-ORAC (Table 2). Total polyphenolic and ascorbic acid content are reported as well.

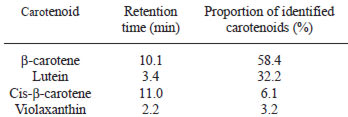
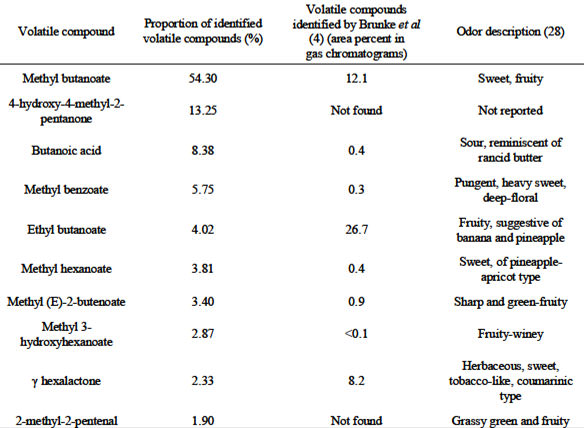
Total carotenoid content was determined in naranjilla whole fruits, pulp, and seedless juice (Table 3). Carotenoid identification in naranjilla whole fruits is presented in Table 4, where ßcarotene was the main carotenoid found. No carotenoid identification in naranjilla fruit has been previously reported. Using GC-MS analysis, 10 volatile compounds were identified in naranjilla pulp (Table 5). In this study, the predominant aroma constituent found in the fruit’s pulp was methyl butanoate, a volatile compound described as sweet and fruity.

When compared with the characteristics of naranjilla fruits from Colombia as reported by Arango et al (2), Romero (20), and Guzmán et al (21), moisture content in the Costa Rican fruits (Table 1) was lower, ash content higher, protein content similar, fat content lower (no fat detected in Costa Rican samples), sugar content higher (sucrose and glucose content higher and fructose content lower compared with samples analyzed by Guzmán et al [21]); and total soluble solids lower. Compared with the Ecuadorian fruit characteristics reported by Dávila (22), total soluble solids of Costa Rican fruits were higher, pH was lower, total sugars were higher, and ash con tent was higher. Morton (5) reported results of analyses performed on fresh naranjilla fruits in Colombia and Ecuador. When compared with these results, Costa Rican fruits showed moisture and fiber contents within the reported range, but they had higher protein and ash, and lower fat content.
The H-ORAC value of naranjilla was higher than those of banana, cantaloupe, honeydew, kiwi fruit, nectarine, pineapple, and watermelon (among others); similar to those of red grapefruit, navel orange, peach, pears, and tangerine; but lower than those of apples, cherry, plums, and all berries (Wu et al [23]). Total polyphenolic content was similar to values found in banana, pineapple, and lemon (24). Ascorbic acid content was lower than values reported by other authors, of 25 (20) and in the range of 31.2-83.7 mg / 100 g (5). Authors have reported contents of total vitamin C of 37.5 (21), 47.5 (22), and 95 mg / 100 g (2) for Colombian and Ecuadorian naranjilla samples.
For Colombian and Ecuadorian naranjilla samples, Morton (5) reported carotene contents of 0.71-2.32 μg / g and Guzmán et al (21) indicated ß-carotene content among Colombian naranjilla samples of 2.212 μg / g (in edible portion). Costa Rican naranjilla seemingly had higher contents. However, total carotenoid content in the edible portion of naranjilla from different locations was lower when compared with fruits with high content (more than 20 μg / g), such as grapefruit, papaya, and nectarine (25). Carotenoid content found in the whole naranjilla fruit (pulp and peel) was similar or higher than those found in vegetables with high total carotenoid content, such as kale, red paprika, and parsley (25). Carotenoid content in naranjilla peel was higher than in its pulp, which was noticeable when the colors of both parts were compared. Carotenoid identification in naranjilla whole fruits indicate that ß-carotene and lutein, two of five carotenoids on which most nutrition research has focused (26), were predominant. ß-carotene, the main carotenoid found in naranjilla, has provitamin A activity and can be converted in the body to retinol (26). Epidemiologic reports suggest that the sum of lutein and zeaxanthin, the retinal carotenoids forming the macular pigment, had the strongest protective effect against neovascular age-related macular degeneration, when dietary intakes of different carotenoids were analyzed (27).
In a previous study, Brunke et al (4) identified 59 components in a solvent extract (80/20, v/v of ether/pentane) of Ecuadorian naranjilla fruit juice after silica gel flash chromatography. Authors also sensorially characterized compounds by sniffing GC. From the list of compounds that Brunke et al (4) identified, with concentrations higher than 0.9% (reported as area percent in gas chromatograms), only ethyl acetate, 3-methylbutyl acetate, ethyl (E)-2-butenoate, ethyl 3-hydroxybutanoate, ethyl 3-hydroxyhexanoate, ethyl 3-acetoxyhexanoate, linalool, and acetic acid were not found in the present study. All of the compounds found in Costa Rican samples were also reported by Brunke et al (4), except for 2-methyl-2-pentenal and 4-hydroxy-4-methyl-2-pentanone.
The chemical characteristics of samples of naranjilla (S. quitoense Lam.) fruits cultivated in Costa Rica were analyzed. The fruits exhibited a chemical composition similar to those previously reported in different locations. Information on chemical composition, antioxidant properties, and volatile constituents may increase this crop’s value in local and regional markets as a fresh fruit and as raw material for processed products.
The authors thank M. Torres, J. Bustos, Y. Chan, and E. Murillo for their technical assistance. They are likewise grateful to Helénica Proverde S. A., the French agency AIRE Développement (grant no. 01-8-CR-27-2), and Ministerio de Ciencia y Tecnología, Comisión de Incentivos para la Ciencia y la Tecnología, Consejo Nacional para Investigaciones Científicas y Tecnológicas (CONICIT), Fondos PROPYME no. FP-009-04 (San José, Costa Rica) for their valuable financial help.
Recibido: 30-09-2008
Aceptado: 15-01-2009