The concentration of polyphenolic compounds, such as flavanols and anthocyanins, and the antioxidant activity in apples (Malus domestica Borkh) seem to differ with cultivar, maturity stage, environmental conditions and the part of the fruit. In this work, the total phenolic, flavanol and anthocyanin content and antioxidant activity were measured in the flesh, whole fruit and peel from apple cultivars Fuji, Epagri COOP24 and Epagri F5P283 cultivated in Southern Brazil. Total phenolic content assayed by Folin-Ciocalteu method, flavanol by modified p-dimethylaminocinnamaldehyde method, anthocyanin content by pH differential method and antioxidant activity measured using ABTS assay. One-way analysis of variance, Tukey’s test and correlation analysis were performed. Within each cultivar, the total phenolic, flavanol and anthocyanin contents and antioxidant activity were highest in the peels, followed by the whole fruit and the flesh. In the peel, whole fruit and flesh the Epagri F5P283 apple had the highest total phenolic contents and the highest total antioxidant activity, while that Epagri COOP24 was highest in flavanols and anthocyanins. Total phenolic content was positively associated with total antioxidant activity in flesh, whole fruit and peel. These results demonstrate that phenolic compounds have a significant contribution to the total antioxidant activity which varies considerably depending of the part of the fruit and of the apple cultivar analyzed.
Key words: Antioxidant activity, phenolics, flavanols, anthocyanins, apple.
La concentración de compuestos polifenoles, como flavanoles y antocianinas, y la actividad antioxidante en manzanas (Malus domestica Borkh) parecen diferir con la cultivar, etapa de madurez, condiciones ambientales y la parte de la fruta. En este trabajo, el contenido de fenoles, flavanoles y antocianinas totales y la actividad antioxidante fueron medidos en fruta entera, pulpa y cáscara de las cultivares de manzana Fuji, Epagri COOP24 y Epagri F5P283 cultivadas en el sur de Brasil. El contenido de fenoles totales se midió con el método Folin-Ciocalteu, flavanoles con el método modificado p-dimetilaminocinamaldehido, antocianinas con el método de diferencia de pH y actividad antioxidante fue medida aplicando el método ABTS. Se realizó análisis de varianza de un factor, prueba de Tukey y análisis de correlación. Dentro de cada cultivar, el contenido de fenoles, flavanoles y antocianinas totales y la actividad antioxidante fueron más alto en las cáscaras, seguidas por la fruta entera y pulpa. En la cáscara, en la fruta entera y pulpa, manzana Epagri F5P283 presentó el contenido de fenoles totales y actividad antioxidante total más alto, mientras que la Epagri COOP24 presentó valores más alto de flavanoles y antocianinas. El contenido de fenoles totales fue asociado positivamente a actividad antioxidante total en la pulpa, fruta entera y cáscara. Estos resultados demuestran que los compuestos polifenoles tienen una contribución significativa a la actividad antioxidante total, los cuales varían considerablemente de acuerdo al de la parte de la fruta y cultivar de manzana analizada.
Palabras clave: Actividad antioxidante, fenoles, flavanoles, antocianinas, manzana.
Department of Food Science and Technology, Federal University of Santa Catarina. Florianópolis, SC, Brazil, Company of Farming Research and Agricultural Extension of Santa Catarina, São Joaquim, Brazil
Apple (Malus domestica Borkh) consumption has been associated with reduced risk of degenerative diseases, such as cancer and cardiovascular diseases (1,2). This association is often attributed to the polyphenolics antioxidants contained in apples, which can protect the human body against oxidative stress by scavenging oxygen free radicals (3). Apples also contain ascorbic acid but explain less than 0.4% of the antioxidant activity, indicating that other factors, such as phenolics, are the main contributors (4). Many studies show that the concentration of phenolic compounds, such as flavanols and anthocyanins in apple differ with cultivar, maturity stage, environmental conditions and the part of the fruit (4-6). According to many authors, the content of total phenolic compounds and the antioxidant activity is particularly high in the peel of apples than whole fruit and in the flesh (4-6). These facts suggested that apple peels may possess more bioactivity than the flesh. Interestingly is the real proportion of peel of the apple fruit (based on its weight) to the whole apple quantity, especially as this part of fruit is frequently discarded as a waste product during apple manufacturing or before eating (7).
Several characterization studies of different parts of apple in cultivars grown in the United States (5), Italy (6), Poland (7) and New Zealand (8) have been carried out on the basis of their phenolic profiles. However, little attention has been given at apple cultivars grown in the Brazil, which constitutes the third largest producer of the South America, participating with about 1.5% of the worldwide production. Therefore, the objective of this study was to compare the total phenolic content, flavanol content, anthocyanin content and antioxidant activity in the whole fruit, flesh and peel from three apple cultivars grown in the Brazil, of which one is commercially important worldwide and two are new selections harvested by Experimental Station of the Agriculture and Animal Husbandry Research Company of the Santa Catarina (known by its Portuguese acronym of EPAGRI). Correlations between the antioxidant activity and total phenolic content were also examined.
Folin-Ciocalteu’s phenol reagent, (+)-catechin, 2,2’-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid) diammonim salt (ABTS), p-dimethylaminocinnamaldehyde (DMACA) and Trolox® were purchased from Sigma Chemical Co. (St. Louis, MO). Gallic acid, potassium peroxydisulfate, potassium chloride and sodium acetate trihydrate were obtained from Vetec (Rio de Janeiro, Brazil). All other chemicals were of analytical grade.
Fifteen fruits of Fuji and two new selections (Epagri COOP24 and Epagri F5P283) were purchased from orchards of Experimental Station of the EPAGRI (São Joaquim, Santa Catarina State, Brazil). All fruits were harvested under standard ripening conditions. Immediately after harvest the fruits were washed with deionized water, towel dried and frozen at -20oC until preparation of the samples. They were peeled, when necessary, with a legume knife. The flesh, whole fruit (flesh + peel), or peels were obtained from five randomly selected apples in each trial to minimize variation. The flesh was the edible portion of the apple without the peel. The whole fruit was the edible portion of the fruit with the amount of flesh and peel maintained in the same proportions as in the whole apple. The peels were the parts of the apple removed by the legume knife, as a thin layer of apple flesh remained adhered to the peel, the peel can be considered as the epidermic zone of the apples.
Five grams (5 g) of apple-frozen peel, apple flesh or whole apple were extracted at room temperature using a Unique 1400A ultrasonic bath (Unique, São Paulo, Brazil). The extraction was performed with 100 mL 80% acetone solution for 15 min. The samples were centrifuged at 1000 x g for 10 min at 5oC and immediately assays were performed in the supernatant recovered. All extracts were made in triplicate.
The total phenolic contents of the apple samples were measured using a modified colorimetric Folin-Ciocalteu method (9). A volume of 2.5 mL of deionized water and 0.100 mL of a known dilution of the extract were added to a volumetric balloon of 10 mL. Folin-Ciocalteu reagent (0.5 mL) was added to the solution and allowed to react for 5 min. Then, 1.5 mL of 20% sodium carbonate solution was aliquoted into the volumetric balloons, and the mixture was completed at 10 mL with deionized water. The color developed for 120 min, and the absorbance was read at 760 nm using a Hewlett-Packard spectrophotometer model HP 8452A (Cheadle Heath, Stockport Cheshire, UK). The measurement was compared to a standard curve of prepared gallic acid solutions (50; 100; 150; 250; 500mg/L) and expressed as milligrams of gallic acid equivalents per 100 g ± Standard Deviation (SD) fresh apple component for the triplicate extracts.
The total flavanol content of the apple samples was estimated using a modified p-dimethylaminocinnamaldehyde (DMACA) method (10) with a standard curve prepared with catechin solutions (2; 4; 8; 10; 12mg/L). Apple extracts (1 mL) was introduced into test tube and added 5 mL DMACA solution (0.1% in 1 N HCl in MeOH). The mixture was vortexed and allowed to react at room temperature for 10 min. Following this, the absorbance at 640 nm was read using a Hewlett-Packard spectrophotometer model HP 8452A (Cheadle Heath, Stockport Cheshire, UK). The results were expressed as milligrams of catechin equivalents per 100 g ± SD fresh apple component for the triplicate extracts.
Monomeric anthocyanin content of the apple peels was measured using a spectophotometric pH differential protocol (11). The anthocyanin content of the flesh and whole apple were not analyzed, as apple flesh of these cultivars does not contain anthocyanins. The apple peel extracts were mixed thoroughly with 0.025 M potassium chloride pH 1 buffer in 1:10 ratio of extract to buffer. The absorbance of the mixture was then measured at 510 and 700 nm using a Hewlett-Packard spectrophotometer model HP 8452A (Cheadle Heath, Stockport Cheshire, UK). The apple peel extracts were then combined similarly with sodium acetate buffer pH 4.5, and the absorbance of these solutions was measured at the same wavelengths. The anthocyanin content was calculated as follows:
Total monomeric anthocyanins (mg/100 g of fresh peel) = A x MW x 1000/(e x C)
where A is absorbance = (A515 - A700)pH 1.0 - (A515 - A700)pH 4.5; MW is molecular weight for cyanidin 3-glucoside = 449.2; e is the molar absorptivity of cyanidin 3-glucoside = 26900; and C is the concentration of the buffer in milligrams per milliliter. Anthocyanin content was expressed as milligrams of cyanidin 3-glucoside equivalents per 100 g of fresh apple peel for the triplicate extracts.
The total antioxidant activity of the apple components was evaluated according with the decolorization of the ABTS radical cation (ABTS·+) as percentage inhibition (12). ABTS was dissolved in water to a 7 mM concentration. ABTS radical cation (ABTS·+) was produced by reacting ABTS stock solution with 2.45 mM potassium persulfate and allowing the mixture (1:1) to stand in the dark at room temperature for 16 h before use. The ABTS·+ working solution was prepared by dissolving ABTS·+ radicalized solution in ethanol (1:50) to an absorbance of 0.700 ± 0.20 at 734 nm. After addition of 1.0 mL of diluted ABTS·+ solution (A734nm = 0.700 ± 0.020) to 0.01 mL of sample the absorbance reading was taken after of 7 min of reaction using a Hewlett-Packard spectrophotometer model HP 8452A (Cheadle Heath, Stockport Cheshire, UK). A standard curve of Trolox (0, 75, 150, 300, 450, 750, 1050μM) was prepared and the percentage inhibition of decolorization calculated as: (1 - Absorbance after 7 min of reaction/Absorbance initial without sample) x 100. The antioxidant activity of samples were expressed as Trolox equivalent antioxidant capacity (TEAC) calculated and expressed using micromoles of Trolox equivalents per 100 g of fresh apple.
All data were reported as mean ± standard deviation of three replicates. The results were compared by one-way analysis of variance (ANOVA) and Tukey’s test were carried out to test any significant differences among the means using STATISTICA 7.0 software (Statsoft Inc., Tulsa, OK, USA). Differences among means at 5% level (P < 0.05) were considered statistically significant. Correlations between the antioxidant activity and total phenolic content were examined using Pearson correlation.
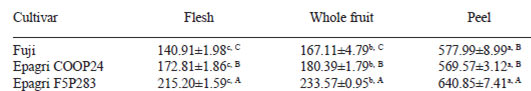
The Table 1 shows that the total phenolic contents were higher in the peels within all three apple cultivars, followed by whole fruit and flesh values. The Epagri F5P283 apple had the highest total phenolic contents in all the parts of the fruit.
The total flavanol contents of the peels were also higher than the whole fruit and flesh values for each cultivar and whole fruits contents were significantly higher than that of the flesh except for Epagri F5P283 apple (Table 2). The Epagri COOP24 apple had the highest total flavanol contents in all the parts of the fruit.
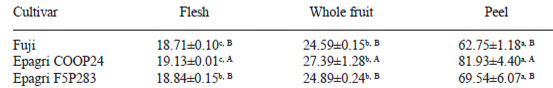
The anthocyanin contents in all apple peels were significantly different (Figure 1). The anthocyanin content of flesh and whole fruit were no analyzed, as apple flesh does not contain anthocyanins.
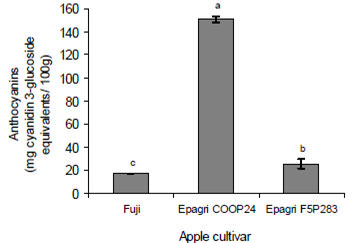
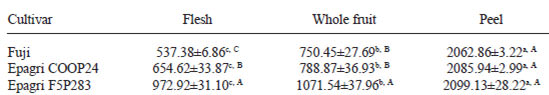
Table 3 describes the total antioxidant activity of three cultivars. In all cultivars, the apple peels had the highest antioxidant activity followed by the whole fruit and the flesh. The Epagri F5P283 apple had the highest total antioxidant activity in flesh, whole fruit and peel.
Total phenolic content was positively associated with total antioxidant activity in flesh (r = 0.9704, P = 0.0001), whole fruit (r = 0.9685, P = 0.0003) and peel (r = 0.8417, P = 0.036), suggesting that phenols have a significant contribution to the total antioxidant capacity of all parts of the apples.
The total phenolic content, flavanol content, anthocyanin content and antioxidant activity vary considerably depending of the part of the fruit and of the apple cultivar analyzed. Comparison of the values obtained in this study with those of other studies suggests similar results although differences in the units reported and spectophotometric standards employed make a direct comparison difficult.
Apple peels possess higher contents of phenolic compounds compared to flesh and whole fruit. A similar trend in the total phenolic content of apple parts was found among studied cultivars in different countries (4-8). The nature and distribution of these phenolics between the flesh and the peel of the apple is also different. Among others, the flesh contains catechins, procyanidins, phloridzin, phloretin glycosides, caffeic acid and chlorogenic acid, the peels possesses all of theses compounds and has additional phenolics not found in the flesh, such as anthocyanins and quercetin glycosides (13-15). Catechin, epicatechin, procyanidins B1 and B2 are main flavanols present in appreciable amounts in both the peels and the flesh (14,15). The total flavanols contents of the peel, flesh and whole apple under investigation were comparable to those previously reported (16,17).
The measured anthocyanin content of the apple peels was related to their appearance. The red color of apple peels is due to the presence of cyanidin 3-galactoside the major anthocyanin present in red or partially red genotypes apples (14,15). The news selection studied Epagri COOP24 and Epagri F5P283 apples were deep red in color and had the most anthocyanins whereas the Fuji apple a bicolored cultivar (bright red with green patches) had significantly less anthocyanins. Similar results were found by other researchers (5,15).
The results of this study were consistent with a number of previous studies which state that apples possessed strong antioxidant capacity and that the value varied between apples cultivars (4,15,18). The Epagri COOP24 and Epagri F5P283 apples, which are resistant cultivars to apple scab compared with the Fuji apple, contain higher content of phenolic compounds and antioxidant activity, despite some results of these two cultivars have similar levels compared to Fuji apple.
Petkovsek et al (18) observed also that some scab resistant apples cultivars, which have a higher natural resistance, possess higher content of total phenolic compounds in comparison with scab susceptible cultivars. The reason why resistant apple cultivars exhibit higher phenolic content and higher antioxidant activity is probably beside genotype also because the trees are exposed to different stress factors in orchard, such as diseases, pests, lack of mineral nutrients, which induce accumulation of phenolic compounds (18,19).
In addition, whole apple tended contain significantly greater total antioxidant activity and total phenolic compared with flesh, suggesting that peel removal may induce a more significant nutrient losses in some cultivars than others. Higher concentration of phenolic compounds and antioxidant activity in peel and whole fruit than flesh was also reported for other apple cultivars (4-6,18,20).
There was a significant positive correlation between total phenolic content and antioxidant activity and in flesh, whole fruit and peel; however, a little weaker in the peel compared to the flesh and whole apple. This is probably because of polyphenolic compounds found in the peel and not in the flesh, as well as the variation in relative proportions measured (15). Moreover, the proportions of compounds within of these parts of the fruit and differences between these might subsequently result in complex changes in activity antioxidant or others bioactivities (15).
The higher total phenolic content in apple fruits resulted in higher total antioxidant activity (4,6,15,18). In contrast, Wolfe et al (5) did not found any correlation between the high antioxidant capacity and level of phenol constituents in apple. Different methods of extraction and analysis, as well as different apple cultivars may contribute to variance in the reported levels of phenolic compounds and antioxidant activity and, as a result, there can be no correlation between these test observed.
In conclusion, the peel and whole apple possess from 1.1 to 4.1 times greater total phenolic content, from 1.2 to 4.9 times greater total flavanol content and from 1.1 to 3.9 times greater total antioxidant activity compared with flesh. Apple peels are often discarded in the production of processed apples products, but clearly they possess high levels of antioxidant and bioactive compounds, which can be used for various purposes in the food, pharmaceutical and cosmetic industry (21). From the nutritional point of view, the results of this study suggest that regular consumption of apple with peel must be recommended to maximize the dietary intake of antioxidant compounds that may have health benefits for consumers such as reduced risk of cardiovascular diseases and cancer.
Authors are grateful to the National Counsel of Technological and Scientific Development for financial support and the Company of Farming Research and Agricultural Extension of Santa Catarina for their collaboration in sample collection.
Recibido: 06-11-2008
Aceptado: 30-01-2009