The purpose of this work was to investigate the influence of energy restriction and vitamin E supplementation on memory, learning, anxiety and spontaneous locomotion in adult rats. Threemonth old male Wistar rats were grouped according to diet: Control (AIN 93-M; n=18), Supplemented (AIN 93-M + 1425 IU all-rac-α-tocopheryl acetate/kg diet; n=22) and Restricted (AIN 93-M with 30% reduction in carbohydrate energy; n=23). Sixteen weeks after, the passive avoidance (PA), elevated plus-maze (EPM) and open field (OF) tests were applied. In the EPM test, the behavioral profile of the supplemented group was characterized by a lower frequency of entries into the open arms (P < 0,026), whereas the restricted group showed a lower frequency of head dipping (P < 0,003). The ratio between the time span of the shocks and the number of attempts were larger for the supplemented than for the non-supplemented animals (P = 0,0474), thus suggesting a delay in learning in the PA test. Taken together, these results suggest that a long-term combination of carbohydrate energy restriction in rats should not cause negative behavioral alterations. Compared with vitamin E supplementation, the restricted diet performed equally or better in rats as an alternate antioxidant diet.
Key words: Diet composition, behavior, energy restriction, vitamin E supplementation, antioxidant diet.
Se investigó la influencia de la restricción energética en comparación a la suplementación con vitamina E en la memoria, aprendizaje, ansiedad y locomoción espontánea en ratas adultas. Machos Wistar de tres meses de edad fueron agrupados de acuerdo a las dietas: Control (AIN 93-M, n=18), Suplementados (AIN 93-M + 1425 UI all-rac-α-tocoferil acetato / kg de dieta; n=22) y Restrictos (AIN 93-M con 30% de restricción energética en los carbohidratos; n=23). Dieciséis semanas después, fueron aplicadas las pruebas de esquiva pasiva (PA), laberinto elevado en cruz (EPM) y de campo abierto (OF). En la prueba de EPM, el grupo suplementado mostró una menor frecuencia de entradas en los corredores abiertos (P < 0,026), mientras el grupo restricto registró menor frecuencia de bajadas de cabeza (P = 0,003). La razón entre la duración de los choques y el número de intentos fue superior para el grupo suplementado, que para los no suplementados (P = 0,0474), lo cual sugiere un leve perjuicio en el aprendizaje de los suplementados, según la prueba de PA. Tomados en conjunto, estos resultados sugieren que la restricción crónica en energía de carbohidratos no provoca alteraciones negativas en el comportamiento de ratas adultas y que sus beneficios pueden superar los obtenidos al suplementar la dieta patrón con vitamina E, como opción de dieta antioxidante.
Palabras clave: Dieta, comportamiento, restricción energética, suplementación con vitamina E, dieta antioxidante.
Food Security and Nutrition Laboratory, State University of Ceará, Fortaleza, CE, Brazil, Faculty of Nutrition, Federal University of Alagoas, Maceió, AL, Brazil, University of São Francisco/Bragança Paulista, SP, Brazil, Department of Food and Nutrition, University of Campinas - Unicamp, Brazil
The impact of the modern environment on well-being, including the diet, is a main concern of health authorities throughout the world. Special emphasis has been placed on the use of antioxidants, whether as part of the diet or in the form of supplements in order to minimize the impact of environmental factors on the general state of health. While no official reference intakes on the vast list of antioxidants have been issued, and as more antioxidants, both synthetic and natural, become available in the market, conscious consumers may prefer to adopt one of several forms of food restriction, among which carbohydrate energy restriction has met some success in the past, as an option to provide themselves with a share of antioxidant benefits.
Energy restriction has been recognized as one of the most consistent dietary manipulations to delay aging in mammals (1-3). Some researchers have focused on the benefits of this practice on the physical performance, learning and information retrieval capacity of old rats (4-6), the learning capacity of young rats (7) and on behavioral tests using experimental models for degenerative diseases (8,9). On the other hand, potentially harmful effects of energy restriction on brain development, such as the effect of protein-energy deficits on cognitive and motor performance in young animals, have also been pointed out (10-13). Although the beneficial effects attributed to energy restriction depend on the adequacy level of all the essential nutrients supplied by the diet and, therefore, defining the diet composition may be of fundamental importance to the experimental design (7), this issue has been poorly addressed.
The health properties of vitamin E have been extensively recognized, including inhibition of smooth-muscle cell proliferation, preservation of endothelial function, inhibition of monocyte-endothelial cell adhesion, inhibition of monocyte reactive oxygen species and cytokine release, and inhibition of platelet adhesion and aggregation. These cellular responses to α-tocopherol are associated with transcriptional and posttranscriptional events. Activation of diacylglycerol kinase and protein phosphatase 2A, and the inhibition of protein kinase C, cyclooxygenase, lipoxygenase, tyrosine kinase 2-phosphorylation, and cytokine release by α-tocopherol are all examples of post-transcriptional regulation that explain the vitamin’s antioxidant actions in the body (14), while its consumption as an antioxidant supplement gains acceptance owing to its very low toxicity (15).
The brain contains high levels of unsaturated lipids and is responsible for 20 to 25% of the body’s oxygen consumption, under conditions that favor the occurrence of oxidative stress. Quantitatively, vitamin E is the predominant encephalic lipophilic antioxidant, whose main function is to protect brain lipids from oxidative damage (16). The physiological decline associated with aging, including psychomotor and cognitive disorders, is considered to be partly the result of an accumulation of molecular oxidative damage (17,18), and this process may be ameliorated by antioxidant therapies, over and above the general recommendation of a plethora of antioxidant foods.
Because the brain tissue is particularly susceptible to oxidative stress we have compared a diet protocol of carbohydrate energy restriction with vitamin E supplementation in Wistar rats and found that both can offer similar protection against oxidative damage to various tissues, except to the brain, where the restricted diet had a significantly better performance (19).
Despite the potential benefits attributed to the above mentioned diet models, the possible repercussions of such interventions for prolonged periods of time on adult animal behavior have not been thoroughly studied and should merit a comparative evaluation. Therefore, the objective of the present study was to examine the nutritional-behavioral relationship, specifically regarding the anxiety level, locomotion activity, learning and memory of adult rats submitted to chronic consumption of energy restricted, as compared to vitamin-E supplemented diets. For this purpose, the classical open field (OF), elevated-plus maze (EPM) and passive avoidance (PA) tests were used.
Sixty-three 21-day old male Wistar rats (Vivarium Center, University of Campinas, Brazil) were housed in collective cages (22 ± 2oC; 12h light-dark cycles) and fed on commercial laboratory chow (Labina, Ralston-Purina do Brasil, Ltd., Campinas, Brazil) ad libitum for eight weeks. At three months of age, the animals were transferred to individual cages to start the experiment and randomly allocated to one of three diet groups. Group C (control; n=18) had free access to the maintenance AIN 93-M diet (20), while group S (supplemented; n=22) was fed diet C ad libitum, but with the addition of 1425 IU of vitamin E (all-rac-α-tocopherol acetate), and group R (restricted; n=23) received a modified C diet at a lower amounts and with less carbohydrate, as detailed in Table 1. Initially, all groups underwent a one-week adaptation period consuming the control diet.
The restricted diet was formulated such that when offering 70% of the amount consumed by group C, the amounts delivered for all nutrients would be the same as for group C, with the exception of the carbohydrates. Consequently, by restricting diet consumption (or energy intake) to 70% of that of the controls, the R group was ingesting 40% less carbohydrate. Diet S, on the other hand, supplied twenty times the amount of vitamin E recommended for the rat (20). In order to keep a closer check on energy consumption, the total energy of the diets was determined using a Parr calorimeter, model 1261/1563 (Moline IL, USA).
The feeding trial was carried out for 16 weeks and the entire study was performed according to the Guide to the Care and Use of Experimental Animals of the University of Campinas, Brazil. The amount of diet to be fed to the R group was determined from the daily average consumed by the C group. Body weight changes were monitored and recorded weekly.
At the end of the 16th week, the effects of the diet on spontaneous motor activity, anxiety level, learning capacity and memory were measured by applying the open field (OF), elevated plus-maze (EPM) and passive avoidance (PA) tests, respectively. The tests were conducted in that sequence throughout a period of two weeks, with intervals of three days between tests. Preceding every test, the freshly fed animals were allowed to stay in the testing environment for 10 minutes in order to remove the effects of the natural search for food or of inhibition due to a new environment. All procedures were manually and video recorded by the researcher from a location visually inaccessible to the subjects.
For the OF test, the instrument consisted of a transparent acrylic chamber (100x100x40cm) with 25 square plots drawn on the floor. The animal was initially placed in one of the corners and, during a 5-min observation period, peripheral and central crossings and rearing, self-washing and defecation activities were registered.
The EPM test was conducted in a roofless, Formica-coated wooden device, positioned 50cm above the laboratory floor and consisting of two open arms (50x10x1cm), with acrylic side walls (1cm), and two closed (50x10x40cm) arms. In order to start the test, the animals were placed at the center of the maze, facing one of the closed arms. Their behavior was observed and recorded for 5min. Standing times in the open and closed arms, self-washing routines, protected (head projected towards the closed arms or central platform) and unprotected (head projected towards open arms), head dipping, listening and both protected and unprotected cautious exploration attempts, were recorded.
The device for the PA test was a box (40x22x30cm) with a metal roof and walls and a floor with stainless steel bars (0.3 cm in diameter, spaced 1.2 cm from each other) connected to a low intensity (1mA) shock generator, to provoke an adverse reaction. A Formica platform (8 x 13 cm), hoisted on stilts, was placed inside the box to one side. The front of the box was of transparent acrylic material to allow for the researcher to see the animal and make a video recording. The test consisted of two phases: acquisition and retention. In the first phase the animal should remain on the platform where he was placed at the start of the test, avoiding the shocks for 200 sec, as a learning parameter. The number of descents (attempts), the time taken to descend from the platform (latency), and the time remaining on the metal bars (receiving shocks) were recorded. Animals remaining on the bars receiving shocks for more than 300 sec were eliminated from the experiment. The second phase, carried out 48 h later, tested retention of the acquired behavior, measuring the time required for the animal to descend from the platform and place his four paws on the bars, without shocks (maximum waiting period, 600 sec). In this phase the number of false descents from the platform was also measured (placing the front paws on the bars and returning to the platform) and the number of rearing movements.
This vitamin was determined by high performance liquid chromatography (HPLC) according to Sharma & Kumar (21), with modifications. The brain tissue was homogenized (50 g/L; 0.05M phosphate buffer, pH 7.4; 90 μM butylhydroxytoluene -BHT) in an ice bucket, using a homogenizer with a Teflon pestle. 400 μL of absolute ethanol were added to the brain homogenate and the mixture vortexed for 1 minute. After centrifugation (12,000 x g for 5 minutes), the tocopherol was recovered in methanol following extraction with n-hexane. Elution from the HPLC equipment (Varian, model 9012, equipped with a 9075 fluorescence detector) was monitored at 290 (excitation) and 330nm (emission). A standard calibration curve for (±)-α-tocopherol was used for quantification, and recorded per unit protein in the tissue. With a few exceptions, the alpha-tocopherol determinations were carried out in half of the animals of each group.
The characteristics of normality of distribution of the data and homogeneity of the variances were analyzed using the tests of Lilliefors and Levene, respectively. Whenever applicable, monovariate ANOVA was performed and differences between groups detected by the tests of Snedecor and Tukey-HSD. When the criteria for parametric analysis were not met, the differences were evaluated by the Kruskal-Wallis test, adopting the Nemenyi test for multiple comparisons. A level of p≤0.05 was selected to indicate minimum significance for all comparisons. The Statistica for Windows program (StatSoft Inc. Tulsa, OK/USA) was used for the statistical analyses.
As expected, the mean weight gain of the restricted group at the end of the experimental period was significantly lower, or approximately 22% of the weight gained by the others (initial mean weights: C = 323.4 ± 25; S = 329.8 ± 28; R = 332.0 ± 26g; final mean weights: C = 467.1 40; S = 473.6 ± 52; R = 364.7 ± 17g; maximal P < 0.001 for changes). The respective final mean brain α-tocopherol concentrations for the control, restricted and supplemented groups were 0.32 0.02, 0.28 ± 0.03 and 0.47 ± 0.05 μg/ mg protein (P between S and the others <0.0001).
The standard Open Field test is commonly used to assess locomotor, exploratory and anxiety-like behavior in laboratory rats. This test is particularly useful in evaluating the effects of anxiolytic and anxiogenic drugs, locomotor responses to drug and as well as behavioral responses to novelty.
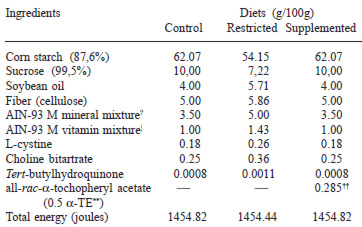
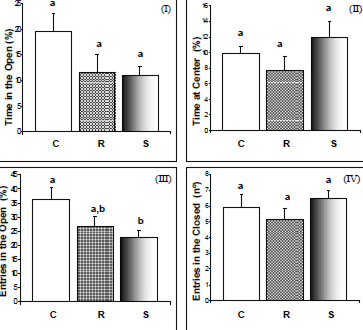
The results shown in Figure 1 indicate no differences in general locomotion activity (central + peripheral crossings) between the supplemented and restricted animals when compared to the control group, or among themselves (F (2.58) = 1.35; P = 0.2678), after a five-minute period. Likewise, no alterations were detected in central roaming (F (2.58) = 1.01; P = 0.3694). Nevertheless, the vitamin-E supplemented group performed a significantly lower number of peripheral crossings during the second minute of the test, with respect to the other two diet groups (F (2.58) = 5.85; P = 0.0048). As time elapsed, however, differences between groups tended to disappear.
There was also no difference between the groups regarding the total number of rearing attempts (C: 27.35 ± 2.41, R: 25.2 ± 1.9 and S: 22.1 ± 2.22; F(2.58) = 1.63; P = 0.2047) or self-washings (C: 0.35 ± 0.19, R: 0.36 ± 0.28 and S: 0.45 ± 0.21; H(2, N= 61) = 2.2414 P = 0.3261), although the number of fecal droppings were significantly greater for the restricted group than for the other two diet groups (C: 1.18 ± 0.32, R: 3.09 ± 0.46 and S: 0.46 ± 0.55; H(2, N= 61) = 6.54 P = 0.0380).
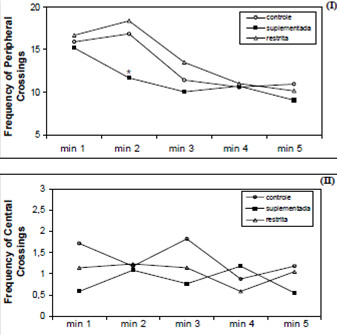
This test is used to assess the anxiety produced by any external or internal factor, such as drug, food or environmental perturbation and is based on the animal’s preference for dark, closed, rather than bright, open spaces. Statistical analysis of the results of the EPM (Figure 2) indicated that the diets under evaluation produced no differences in the general parameters analyzed by this anxiety test. Nevertheless, excluding any zero-entry events (Figure 3), the analysis showed that animals of the supplemented group entered the open arms a lower number of times, in comparison to the controls (F (2.38) = 4.04; P < 0.026), suggesting they felt less confident when being in the open. A significantly lower number of head dips (C: 8.0 ± 0.98, R: 3.29 ± 0.53 and S: 6.64 ± 1.34; F (2.38) = 6.79; P < 0.003), and unprotected dips (C: 5.42 ± 0.1, R: 1.93 ± 0.47 and S: 3.14 ± 0.88; H(2.0 N =41) = 7.48; P = 0.02) were also evident for the restricted group, as compared to the control. Head dipping is another indication of self confidence and desire to explore. With respect to listening activities (rearing movements) (C: 0.94 ± 0.3, R: 0.65 ± 0.2 and S: 0.59 ± 0.2) and cautious exploration (C: 2.06 ± 0.6, R: 1.39 ± 0.3 an S: 2.23 ± 0.4), no significant differences were found between the groups under study (P > 0.05).
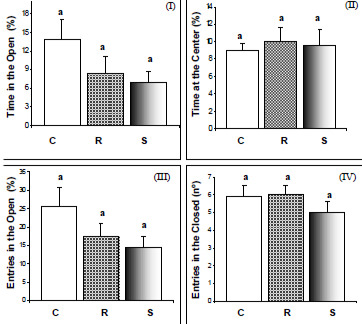
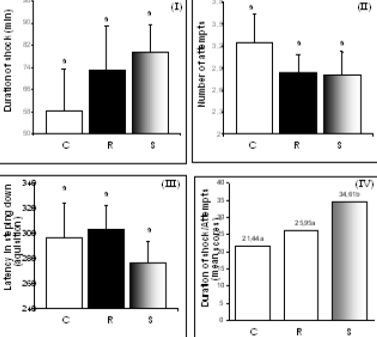
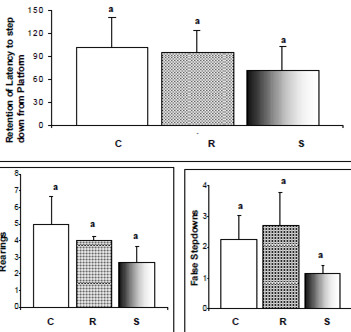
Passive-avoidance response (PAR) is extensively used for the screening of drugs affecting learning and memory. The test involves training rodents to avoid punishment (normally an electric shock) by curbing a normal behavior (such as the exploratory behavior). At specified intervals after training, the animals are tested again for retention of such learning. The conventional and the most widely employed parameters are step-down latency (SDL) and step-down errors (SDE). In order to test the consistency of the learned behavior, we introduced an extra parameter, namely, the total time spent by the animal in the shock zone (TSZ). The parameters of time-exposure to shock and number of attempts recorded during the avoidance response-acquiring phase (Figure 4) showed no difference between the groups when analyzed individually (F(2.57) = 0.43; P = 0.6529 and H(2, N = 60) = 0.88; P = 0.6452, respectively). Since the means did not differ considerably, a new parameter was calculated by taking the ratio between the former two parameters (Figure 4-IV), and this time the ratios showed a significant difference between the supplemented and both control and restricted animals. One rat from the control group and another from the supplemented group were excluded during the first phase of the test for spending longer than 300 seconds on the bars, without acquiring the avoidance response. In the second phase of the test, no statistical difference was found between the different diet groups with respect to the time spent on the platform (H(2, N = 61) = 0.88; P = 0.6441), suggesting a similarity in the retention of the learned behavior. Alterations were also not detected with respect to rearing and false descent movements (SDE; Figure 5).
The majority of studies on the effect of diet on animal behavior have featured the repercussions of malnutrition and undernutrition on growth and development (11,13,22-25), while others have evaluated the effects of isolated nutritional factors, mainly regarding the benefic action on chronic diseases and aging (2,5,17,18,26-30). Meanwhile, other works have dealt with the influence of energy restriction on development and reproduction (7,8), as well as alternative antioxidant diets (17). This work sought to compare the impact of the chronic administration of two antioxidant dietary regimes, energy restriction (in both carbohydrate content and total energy intake restriction) and vitamin E supplementation on rat behavior.
Upon confrontation of the results obtained in the open field test of the present study with those of the behavioral standard designed by Huston & Bures (31) for adult rats, the observed performance was similar to that described in the literature, with no differences in general activity found between the two diet models investigated. The supplemented animals, however, explored less the peripheral squares between the first and second minute of observation (Figure 1) and this could be somewhat relevant if we were to consider quick responses, but not if we consider the total time interval of the test, when no difference was found among the groups (C = 72.4 ± 6; R = 74.9 ± 8; S = 60.7 ± 6 F(2.58) = 1.35; P = 0.2678). Since the control and the supplemented groups bore similar body weights and the controls showed a tendency to normal productive locomotion, the possibility that the supplemented animals were discouraged from any greater exploratory activity because of their weight had to be discarded. Therefore, it appears that supplementing the standard diet with extra doses of vitamin E may have resulted in a partial loss of exploratory desire of the animals so treated.
Similarly, Mcdonald et al. (17) did not observe significant improvement of the psychomotor performance of 24-month old mice fed antioxidant diets containing either vitamin E, coenzyme Q or a mixture of both for 14 weeks. Interestingly, these authors did notice a moderate impairment in the performance at the initial phase of coordinated running, with either the vitamin alone or in combination with the coenzyme. Sumien et al. (32) in turn have reported deleterious effects in vitamin-E supplemented aged mice, yet not in young ones, when submitted to tests demanding coordination and fast responses, after a short-term supplementation with a dose similar to the one used in this work. Contrasting with our experiment, the supplementation by Sumien et al. (32) and Mcdonald et al. (17) started when the animals were at least 20 months old.
On the other hand, Shukitt-Hale et al. (30) reported beneficial effects of some antioxidant diets tested by the group, including age-related cognitive behavioral deficits and changes in neuronal signal transduction due to vitamin E supplementation. One of the diets tested was a blueberry based diet that reverted deficits in motor performance. In another study involving the administration of diets with various concentrations of vitamin E and C, plus selenium, fed to 12-month old mice for six months, Richwine et al. (18), also concluded that the diet containing high levels of antioxidants showed to be beneficial, considering that they improved the psychomotor coordination of the 18-month old animals in the rod walking test. The results of our study, therefore, should not necessarily agree with those discussed above for our objective was to assess the response of rats with clearly defined diets and without the intervention of complex mixtures of foods or the cocktail effect of multiple nutrient supplementation. It is still possible, however, that clear-cut beneficial behavioral effects of such antioxidant diets are somewhat agerelated, being more evident at the onset of the psychomotor decline, rather than at an early age when target features of behavior can be overridden by either strong adaptive metabolic pathways of the young, or later on, by irreversible metabolic developments that have already been installed.
It is worth mentioning that apart from establishing the spontaneous activity pattern of the animal, the open field test can be used as a simple evaluation of the emotional state. It is known that changes in emotional state are accompanied by various vegetative phenomena, such as changes in the heart rate, voltaic response of the hair and dilatation of the pupils. In addition to these features, one can evaluate the autonomic defecation function, considering that animals that roam less and defecate more are referred to as being more emotionally affected than those with the oposite behavior (31).
Generally and under standard conditions, the behavior of adult rats shows a high negative correlation between defecation and exploration of the central squares. Although the restricted animals excreted a greater number of fecal droppings, it was surprising to find no correlation between this parameter and central roaming. Harrison & Archer (1) used this test in various assays with mice and observed a greater activity in adult and elderly animals fed a restricted-energy diet, as compared to controls with free access to food. However, more recently, Wu and coworkers reported no detectable differences in the exploratory activity of mice after three (7) and six (8) months consuming diets with 20 and 35% food restriction, respectively. Therfore, it was not surprising to find only a loose correlation between the defecation parameter and the central roaming activity.
The elevated-plus maze is a classical test that measures the degree of anxiety of the animal as being directly proportional to the length of time it spends in the enclosed arms, or avoiding being exposed in the open. With regard to the behavioral features analyzed by the EPM, we observed that, as with the open field results, it was not possible to detect any differences in exploratory or other ethological performance of the adult animal between energy restriction and vitamin E supplementation during the 16-week period, indicating at first glance that neither of the antioxidant dietary models was likely to interfere with the anxiety levels of the animals by this criterion.
It is important to observe, however, that a considerable percentage of the animals, similar for all three groups, did not explore the open arms of the maze at all (C=30%, R= 35%, S=35%), thereby increasing the dispersion of the data in all groups. For this reason the data were further analyzed, this time excluding the non-responsive animals (Figure 3). Upon new analysis, the vitamin-E supplemented group showed a lower percentage of entries in the open arms when compared to the control, thus suggesting an anxiety response in this group, about 28% greater than in the control. The restricted group meanwhile, did not differ from the control. The reason why none of the groups showed a fuller responsiveness is not known. Reactive oxygen species have been critically implicated in various types of pain, including neuropathic and inflamatory pain, in which vitamin E seems to play an analgesic role (33). If this were the case, a slight attitude of indifference could have lowered the response to the test by these animals.
Rodgers & Cole (34), however, examined the behavioral response of mice in the EPM test soon after an experience of aggression, and demonstrated the importance of social stress factors in the production of analgesia. The anxiety response effect of social competition was partially reproduced in mice simply by exposing them to the smell of an aggressive male, producing a reduction in the total number of entries and rearing movements, and also in the percentage of entries into the open arms, as well as the time spent there. By establishing a parallel with the present study, and interpreting the prolonged isolation of the animals as a factor of stress (25), we should arrive at the oposite conclusion because the excess vitamin should confer an additional edge of relaxation to the supplemented group. Therfore, it seems reasonable to suspect that a factor, other than solely analgesia, may have contributed to the lower number of entries of the supplemented rats in the open arms.
An analysis of the results obtained in some studies evaluating the learning capacity and memory of animals fed diets with different compositions showed that for the inhibitory passive avoidance the behavioral changes more likely to occur in undernourished animals were lower thresholds to the perception of shock, shorter avoidance latency and greater resistance to extinguishing the avoidance response (12,35).
Additionally, Goodrick et al. (36) asserted that once learned, the information remained available for an extensive period of time. These researchers observed a substantial retention of learning up to 45 days after the avoidance test was performed in Wistar rats, both young and old.
In general, undernutrition does not affect acquisition of the avoidance response, but animals in this condition normally require a greater number of attempts on the springboard and in the come-and-go box (12). In our experiment, results of the retention phase (performed 48h later) suggested that neither high levels of vitamin E supplementation or 30% energy restriction for the prolonged period of the study influenced memory recall of the adult rats because in all cases the acquired response was evidently retained.
Moreover, Socci et al. (5), upon administration of a combination of intra-peritoneal injections of phenyl-α-tertbutylnitrone (PBN; 32 mg/kg body weight) and α-tocopherol (200 mg/kg body weight) for approximately 4 to 5 months, in 24 month-old rats, showed that the animals treated with antioxidants had a greater learning acquisition rate. In the experiment of McDonald et al. (17) the authors reported a beneficial effect on learning and memory consolidation of aged rats from feeding a combination of vitamin E and coenzyme Q, but this effect was not detected when the supplemental nutrients were administered individually. In such case, it was hypothesized that a synergic action could have contributed to the final antioxidant effect of the mixture.
In the passive avoidance test, one could think that the vitamin E supplement could have had some influence on the animals’ performance because of an induced opioid-type analgesia. This mechanism has been little investigated, but there is evidence to this effect in studies published by Kryzhanovskii et al. (37,38) in the former Soviet Union, where an analgesic action was perceived when vitamin E was administered to women with dysmenorrhea.
The effect of 100 mg α-tocopherol acetate, via intra-muscular injection, on reducing pain, was effective 15-20 minutes after application. When a dose of 500-600 mg was applied during two consecutive menstrual cycles, a residual effect of up to six months after administration was found. The authors showed evidence of a possible involvement of the endogenous opioid system in the analgesic effect induced, mobilizing β-endorphin from the pituitary gland. The involvement of this system was confirmed by injecting naloxone – an antagonist of morphine receptors and endogenous opioid peptides. The analgesic effect of the repeated administration of α-tocopherol has been also blocked after injecting naloxone (37,38). Direct evidence for this association, however, could be sought in future investigations.
In spite of all these considerations, it appears as though any possible handicap associated with the supplemented diet, in relation to energy restriction, may be of such complex nature that the present tests are not designed to determine if the vitamin’s analgesic property is involved.
By the application of three classical behavioral tests we can conclude that young adult rats submitted to a chronic (four month) carbohydrate-energy restricted diet, suffered no detectable alterations in their normal behavioral characteristics, as evaluated by locomotor activities and various manifestations of anxiety, in comparison to the group fed a standard diet ad libitum. The carbohydrate-energy restricted animals performed equally or somewhat better than cohorts that received vitamin E supplementation at a level 20 times the recommended intake. Therefore, it can be said that in addition to performing better than vitamin-E supplementation as a long term antioxidant diet, the prolonged practice of energy restriction, combined with specific carbohydrate limitation, may not affect the normal behavior of the adult rat. Albeit a possible analgesic effect could have played a role in the response of the vitamin-E supplemented group, we think this is an issue that should deserve further investigation.
The authors thank the Brazilian agencies CNPq and CAPES for the doctoral fellowships and to Prof. Dr. Cyro Rêgo Cabral Jr., of the School of Nutrition, Federal University of Alagoas for his kind support with the statistical treatment of the data.
Recibido: 01-09-2008
Aceptado: 23-05-2009