Honey is the most popular bee product used by man, with nutritional and medicinal purposes. Its great diversity is attributed to numerous factors (bee type, visited flora, environment, and management). The quality of honey is controlled with routine parameters (free acidity, diastase activity, reducing sugars, ash, water, hydroxymethyfurfural, and sucrose contents). Besides the biochemical quality control, a functional profile is also important for pharmacological applications. In this work, bioactive indicators such as the antioxidant activity, flavonoid and polyphenol contents were evaluated by spectrophotometry, and correlated to the content of six bioelements (Ca, Cu, Fe, Mg, Mn, Zn) measured by atomic absorption spectroscopy, tandem FI-FAAS, in 14 unifloral Czech honeys. The antioxidant activity was 43.13 ± 53.72 μmoles TEAC/100 g honey. The flavonoid content was 5.18 ± 4.19 mg QE/100 g, and the polyphenol content was 45.38 ± 27.20 mg GAE/100 g. Buckwheat honey showed the highest values for these indicators of bioactivity, the acacia honeys the lowest, and the rest of the honeys were comprised between both of them. Honey content of bioelements was 138.19 ± 55.57 ppm Ca (min 77.11 – max 261.65), 0.33 ± 0.41 ppm Cu (min 0.00 – max 1.37), 2.95 ± 1.10 ppm Fe (min 1.34 – max 5.36), 35.08 ± 29.59 ppm Mg (min 8.76 – 128.06), 4.93 ± 3.99 ppm Mn (min 0.34 – max 11.31), 1.07 ± 0.56 ppm Zn (min 0.49 – max 2.52). The antioxidant activity of honey was significantly correlated to its content of cupper, iron, magnesium, manganese and zinc, but was not correlated to calcium.
Key words: Antioxidant capacity, bioelements, flavonoids, polyphenols, unifloral honey.
La miel de abejas es el producto apícola más popular utilizado por el hombre, con fines nutricionales y medicinales. Su gran diversidad se atribuye a numerosos factores (tipo de abeja, flora visitada, ambiente, y manejo). La calidad de la miel se controla con parámetros de rutina: acidez libre, actividad de la diastasa, azúcares reductores, cenizas, humedad, hidroximetilfurfural, sacarosa aparente. Junto con el control de calidad bioquímica, también es importante su perfil funcional para aplicaciones farmacológicas. En este trabajo se evaluaron indicadores de bioactividad como la actividad antioxidante, el contenido de flavonoides y de polifenoles por espectrofotometría, y se correlacionaron con el contenido de seis bioelementos (Ca, Cu, Fe, Mg, Mn, Zn) determinados por espectroscopía de absorción atómica, IF acoplada a EAA con llama, en 14 mieles uniflorales checas. La actividad antioxidante fue 43.13 ± 53.72 μmoles TEAC/100 g miel. El contenido de flavonoides fue 5.18 ± 4.19 mg EQ/100 g, y el de polifenoles fue 45.38 ± 27.20 mg EAG/100 g. La miel de trigo sarraceno mostró los mayores valores en estos indicadores de bioactividad, las mieles de acacia los más bajos, y en el resto de las mieles estuvieron comprendidos entre ambos. El contenido de bioelementos en la miel fue 138.19 ± 55.57 ppm Ca (min 77.11 – max 261.65), 0.33 ± 0.41 ppm Cu (min 0.00 – max 1.37), 2.95 ± 1.10 ppm Fe (min 1.34 – max 5.36), 35.08 ± 29.59 ppm Mg (min 8.76 – 128.06), 4.93 ± 3.99 ppm Mn (min 0.34 – max 11.31), 1.07 ± 0.56 ppm Zn (min 0.49 – max 2.52). La actividad antioxidante de la miel se correlacionó significativamente con su contenido de cobre, hierro, magnesio, manganeso y zinc, pero no se correlacionó con el contenido de calcio.
Palabras clave: Actividad antioxidante, bioelementos, flavonoides, polifenoles, mieles uniflorales.
Departamento Ciencia de los Alimentos, Departamento de Bioquímica, Departamento de Química, Departamento de Farmacología y Toxicología. Universidad de Los Andes, Mérida-Venezuela
Honey is the product obtained from secretions of living parts of plants or excretions of plant sucking insects, and the activities of the bees to collect the sugary fluids, combine them with their own substances, transform, deposit, dehydrate, store, and leave in the honey comb to ripen and mature (1). Although honey is reported to contain about 181 substances (2), it needs a simple description for quality control purposes. Therefore, according to the Codex Alimentarius Commission (CODEX, 2001) “honey consists essentially of different sugars, predominantly fructose and glucose as well as other substances, such as organic acids, enzymes and solid particles derived from honey collection”. In this description, water, which is the second main component of honey, is missing (3), and therefore needs to be added and considered in analytical procedures.
All over the world, honey is considered an ingredient of traditional and complementary/alternative medicine. Scientific support is emerging with several publications on the diversity of its therapeutic effectiveness (4,5). There are a few reports about the effectiveness of honey in gastric ulcers or gastrointestinal disorders in humans (6). Honey has been reported to be effective in healing of wounds and burns (7). Currently, there is overwhelming evidence that free radicals cause oxidative damage to lipids, proteins, carbohydrates and nucleic acids. Therefore, reactive oxygen species (ROS) such as the superoxide anion (O2 •-), the hydroxyl radical (OH•), and the lipid peroxyl radical (LOO•) might lead to many biological complications including carcinogenesis, mutagenesis, aging, atherosclerosis and neurodegenerative diseases (8). A large variety of pathologies has been related to ROS. Therefore it is quite important to find new antioxidants that could lessen the deleterious effects of ROS on the organism. These free radicals are removed by various forms of antioxidants. In general, the term antioxidant is any substance that when present at low concentrations compared to those of an oxidizable substrate, significantly delays or prevents oxidation of that substrate, including various types of molecules found in vivo (9). Natural antioxidants can be phenolic compounds (tocopherol, flavonoids, and phenolic acids), nitrogen compounds (alkaloids, chlorophyl derivatives, amino acids, and amines), or carotenoids as well as ascorbic acid (10-12).
Bioelements of honey are studied for their nutritional importance, as indicators of geographical origin and industrial pollution (13-16). The aim of this work was to study what bioelements are correlated to the antioxidant capacity of eight unifloral honeys from Apis mellifera L. For this purpose, we measured their antioxidant capacity, flavonoids, polyphenols, and the content of calcium, cupper, iron, magnesium, manganese and zinc.
The fourteen unifloral honeys harvested in the Czech Republic are indicated in Table 1. These honeys were not heated prior to packaging in year 2006. The botanical origin of the samples was confirmed by qualitative melissopalynological analysis, with more than 45% pollen counts of the dominant pollen type (17).

Honeys were viscous liquids or solidified after crystallization. Acacia honey is very light yellow, remains liquid and has a very sweet taste. Buckwheat honey is reddish brown and has a strong animal aroma. Heather honey. Lime honey is golden brown and has a strong medicinal aroma. Rape honey has fine crystals and is frequently named “bee lard” because his color and texture are creamy. Raspberry honey also crystallizes, has light color and very sweet taste. Sunflower honey is yellow, sweet taste and sour fruit aroma. Pine honeydew honey is pale brown, has fine crystals and menthol aroma.
The total antioxidant activity was estimated as Trolox antioxidant capacity (TEAC) after the decolorization of the ABTS+? radical cation (18). A mixture 1:1 of 7 mM ABTS (Sigma-Aldrich, USA) and 4.9 mM ammonium persulfate was prepared, covered with foil for 16 h, and diluted up to an absorbance of 0.7 ± 0.2 at 734 nm, reached with approximately 40 μL of reagent + 760 μL ethanol. To this mixture, 10 μL of the diluted honey (0.1 g of honey plus 1 mL of 20% v/v ethanol) were added, shaken vigorously and absorbance was recorded at 734 nm after 6 min using a UV/VIS Perkin-Elmer Lambda 3B spectrophotometer. A calibration curve with 0.625-1.25-2.5 mM Trolox (Sigma-Aldrich, USA) was used to measure the percentage of decolorization, to estimate μM Trolox equivalents/100 g honey.
Total flavonoid contents in the sample were determined by the method of Woisky and Salatino (19), with minor modifications. To 0.1 mL of the honey sample solution, 0.5 mL of 20 mg/mL AlCl3 (Fischer Scientific, USA) ethanol solution were added. After 60 min at 25°C, the absorbance was measured at 420 nm. Total flavonoids were calculated as mg quercetin (Sigma-Aldrich, Germany) equivalents QE/100 g honey from a calibration curve.
Total polyphenol contents in sample were determined after the Folin-Ciocalteu colorimetric method (20). Sample solution (0.1 mL) was mixed with 0.5 mL of the Folin-Ciocalteu reagent (Sigma-Aldrich, USA) and 0.4 mL of 7.5% Na2CO3 (IQE, Venezuela), and the absorbance was measured at 765 nm after 10 min at 37°C. Total polyphenol contents were expressed as mg gallic acid (Sigma-Aldrich, Germany) equivalents GAE/100 g honey.
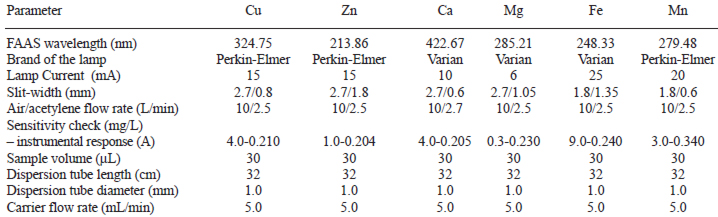
A flame atomic absorption system (FAAS) Perkin Elmer, model Analyst 200 was coupled to a system of continuous flow injection (FI) analysis consisting in a peristaltic pump (Gilson, model Miniplus 3, water with specific resistance 18 MÙcm as carrier flow, an injector and Tygon tubes. The radiation source was a Perkin Elmer or Varian specific catodic lamp for each element. Stock and honey dilutions were injected with a 100 μL Perkin Elmer syringe.
Analytical grade reagents (Merck) were used unless other brand is informed. All solutions were prepared with deionized bidistilled water with specific resistivity of 18 MÙ.cm which was obtained with a Millipore Milli-Q Plus system. This water was also the carrier flow in the FI system.
Honey matrix interference studies were done by the standard addition technique, with variable volume of the stock and fixed final volume. No significant differences (p < 0.05) were found between the slopes of the simple calibration curve and the addition graphs. Therefore it was concluded that the honey had no effect on the measurements, and the simple calibration curve was used.
The percentage of relative standard deviation (% RSD) to meet the recommended interantional interval (< 5%) measured the precision of the method.
All glassware was washed with liquid detergent and abundant water, rinsed with a 20% (v/v) nitric acid solution, and washed six times with deionized bidistilled water of 18 MÙ.cm resistivity.
Diluted stock aqueous solutions for calcium, cupper, iron, magnesium, manganese and zinc were prepared daily in 100 mL clean flasks.
Honey (3.0 ± 0.1 g) was covered with a minimum volume of hydrogen peroxide 35% Riedel-de Haen (1 mL), digested at room temperature (25°C) until complete dilution (approximately 24 h), diluted to a final volume of 5 mL and refrigerated (4°C) until analysis.
In Table 2 are shown the experimental conditions of the analytical method used to determine bioelements in honey.
The recovery study of each analyte was done to validate the accuracy of the method. The absorbance of the honey sample and the stock bioelement were measured. Percentage recovery values were within the recommended international standards (100 ± 5%).
In Table 3 are listed the analytical characteristics of the FAAS-FIA system to measure Ca, Cu, Fe, Mg, Mn and Zn in honey.
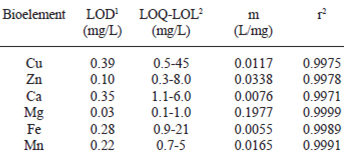
SPSS 12.0 software (21) was used to calculate mean ± SD for all variables, and to correlate variables.
Data from Table 4 show the antioxidant activity and contents of flavonoids, polyphenols and elements in each type of unifloral honey, with highlighted minimum and maximum values in each parameter. The average antioxidant activity of all unifloral honeys was 43.13 ± 53.72 μmolesTEAC/100 g honey. The average flavonoid content was 5.18 ± 4.19 mg QE/100 g, and the average polyphenol content was 45.38 ± 27.20 mg GAE/100 g. Fir honeydew honey showed the highest values for antioxidant activity, but higher values of flavonoid and polyphenols content were foun in buckwheat honey. The acacia honey has the lowest values of the three indicators of bioactivity, and the rest of the honeys were between them.
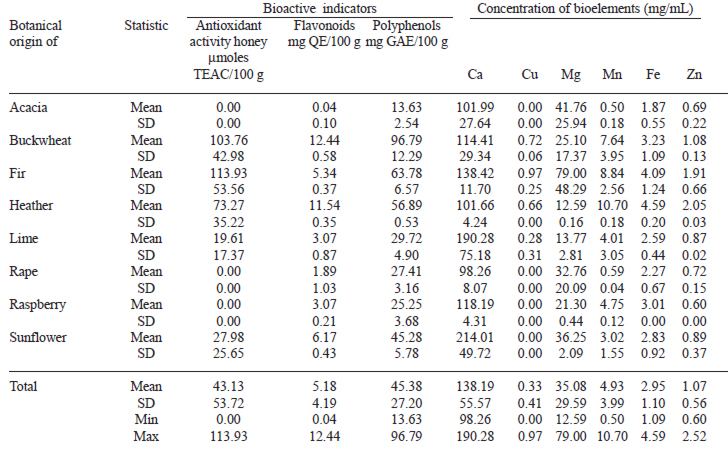
Honey content of bioelements was 138.19 ± 55.57 ppm Ca (min 77.11 – max 261.65), 0.33 ± 0.41 ppm Cu (min 0.00 – max 1.37), 2.95 ± 1.10 ppm Fe (min 1.34 – max 5.36), 35.08 ± 29.59 ppm Mg (min 8.76 – 128.06), 4.93 ± 3.99 ppm Mn (min 0.34 – max 11.31), 1.07 ± 0.56 ppm Zn (min 0.49 – max 2.52). Pearson correlations are shown in Table 5.
This work is a contribution to characterize a bioactive profile of Czech unifloral acacia, buckwheat, heather, lime, rape, raspberry, sunflower and fir honeydew honey.
The origin of bioelements is not well understood. In a work with Portuguese honeys, poor correlation was found between honey bioelements, with soil, tree bark and lichens (22). In another study with Italian honeys, Cu and Fe could not be traced back and variations were attributed to the heterogenous nature of materials collected by bees (23). Predicting the species and the fractions of the bioelements measured in honey, is even more challenging. These elements could be present in enzymes, diluted salts, or participate in other systems like Redox: 1. Ca(II) is like a bridge in the coordination geometries and controls protein fold by carbonyle interaction. 2. Cu varies its oxidation state from Cu(I) to Cu(II) and participates in oxidases, electronic carriers and monooxigenases. 3. Fe is a promoter of the Fenton reaction, Fe(II) and Fe(III) electron transfer. 4. Mg(II) is in the clorophyll porphyrin ring and stabilizes nucleic acids. 5. The enzymes superoxide dismutase and catalase contains Mn, also Mn mononuclear and polinuclear complexes have a biological function in photosynthesis. 6. Zn(II) is the metallic center of many metalloenzymes (polymerases, ligases, transferases, alcaline fosfatases, aminopeptidases, collagenases, hydrolases), nucleic acid and lipids (24).
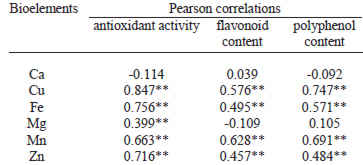
Although honey is not a major source of antioxidants and bioelements in the human diet, it has the ability to scavenge free radicals (25), and to inhibit chemiluminescence in a xanthine-xanthine oxidase-luminol system that works via generation of superoxide radicals (26). Honey also has the potential to exert an antioxidant action by the inhibition of the formation of free radicals in the first place. Honey flavonoids and other polyphenols will do that (27). The phytochemical profile of honey is known for the content of flavonoids and phenolic acids, which operate as antioxidants. The water soluble antioxidant fraction contains chrysin, quercetin, kaempferol, galangin, pinobanksin, pinocembrin, additional to vitamin C and catalase, creating an antioxidant system unique to honey (28). The unifloral Czech honeys studied here revealed that the antioxidant activity is significantly correlated to their content of cupper, iron, magnesium, manganese and zinc, as well as their content of flavonoids and polyphenols (See Table 5). Fractionation by solid phase extraction (SPE) separated a major cationic class (60.9 – 88.9%), hydrophobic, and residual metal species of cupper, iron, manganese and zinc, with suggested organic complexation of these metals in honey (29, 30). Aminoacids, aroma compounds, enzymes, flavonoids, organic acids, proteins, polyphenols and vitamins are able to form a transition metal complex with donor atoms of oxygen, nitrogen or sulphur. The residual metal species are likely to form stable anionic or neutral complexes with organic acids and aminoacids, whereas the hydrophobic species complexed with phenolics reduce the metal absorbablity from food (31). However, the oxidative path of Fenton reactions can be stopped by honey antioxidants complexing with the catalyst Fe2+.
Calcium concentration was not correlated to the antioxidant activity, flavonoid and polyphenol contents of honey. In a previous work, metals and antioxidant activity of Turkish red pine honey were measured by plasma and DPPH respectively, but they only correlated polyphenols and antioxidant activity (32), therefore this is the first time bioelements are correlated to the antioxidant activity of honey.
Additional to metal profiles, further studies should address to the different organic ligands of the honey matrix needed to predict the nutritional and toxic properties caused by the set of metal species having biological roles. The function of these metals in the bee is another aspect to trace back the presence of different species in honey. For example, cellular redox is crucial for bee olfactory processing, and modulation is achieved by iron chelation to prevent ROS mediated oxidative stress (33).
To Mr. Bronislav Gruna and to the Bee Research Institute at Dol, Eng. Michal Bednar and Prof. Dalibor Titera, for providing the unifloral acacia, buckwheat, heather, lime, rape, raspberry, sunflower and honeydew honey samples from the Czech Republic. To financial support received from CDCHTULA project FA-458-09-03-B, and CVI-ADG-FA-04-97.
Recibido: 04-08-2010
Aceptado: 15-11-2010