Deep-fat frying is widely used in food industries because of its low cost and high demand, since it produces convenient food of high acceptability. The process is based on the oil-food interaction at high temperatures, which cooks and dehydrates the food, leading to physical and chemical changes, such as starch gelatinization, protein denaturation, flavoring and color production via Maillard reaction. Some food and oil compounds are lost in the frying process, and potentially toxic compounds are developed in the oxidized oil. Although widely studied, many of these compounds have not been fully identified. The purpose of this study was to review literature findings on changes in food caused by the frying process.
Key words: Composition of food, frying oil, sensory changes
O processo de fritura é amplamente utilizado em indústria de alimentos devido ao baixo custo e alta demanda pela praticidade e grande aceitação. O processo é baseado na interação óleo-alimento a altas temperaturas, que cozinha o alimento e desidrata, levando a alterações físicas e químicas assim como a gelatinização do amido, desnaturação de proteínas, aroma e produção de cor através da reação de Maillard. Alguns compostos presentes nos alimentos e no óleo são perdidos no processo de fritura, e componentes potencialmente tóxicos são desenvolvidos nos óleos oxidados. Embora diversos tenha havido avanços na identificação desses componentes, muitos ainda não foram identificados. A proposta desse trabalho foi avaliar as alterações nos alimentos causadas pelo processo de fritura.
Palavras chave: Composição do alimento, óleo de fritura, alterações sensoriais
Department of Food Engineering, University of São Paulo.
Pirassununga- SP, Brazil.
Frying is one of the oldest food processing methods. Its popularity is related to the ease and speed of food preparation and sensory characteristics, such as unique flavor and taste (1). It is a cheap and fast process of simultaneous heat and mass transfer that changes the sensory and nutritional characteristics, as result of complex interactions between food and oil (2).
Frying is an efficient cooking method because it is a result of high temperature and fast heat transfer (3). The oil which the food is immersed, acts like a heattransferring compound. The process has a preserving action caused by thermal destruction of microorganisms, enzymes and reduction of water activity on the surface of the food (4).
Changes in food and oil depends on the characteristics of the food, oil type, surface/volume ratio of the oil, rate of air incorporation of into the oil, temperature, heating process, length of immersion and the kind of material the frying container is made of. Additionally, the longer the oil is used, greater is the induction of adverse reactions. Extended exposure of oil to high temperatures and atmospheric air can generate highly oxidized, potentially toxic products (5).
In foods, some reactions that affect the nutritional quality may occur (6,7). The frying process relies on high temperatures and can changes the structure of labile nutrients, such as proteins, vitamins and antioxidants. Some water-soluble molecules, such as ascorbic acid can be lost during the water evaporation.
Furthermore, the intake of fried foodstuffs increases the consumption of fats and oils (8). High consumption of food rich in fat has been linked with several metabolic diseases, including obesity, which is a public health problem (9).
In spite of the numerous studies on changes in the oil during the frying process, little information has been compiled about the changes in food. Therefore, the purpose of this review was to highlight important knowledge on the changes that occur within the food during the frying process.
Simultaneous mass and heat transfer by hot oil modifies the food surface, forming a crust that preserves flavors and retains part of the juiciness of the food while it is cooked, making chewing and digestion easier (1).
Deep-fat frying oil reaches 175°C in average, ranging from 150 to 200°C (10). Temperature in the frying is very heterogeneous: the highest temperatures (which are close to oil temperatures) are observed in the peripheral region of the food while the core of the food, rich in water, shows temperatures around 100oC (usually between 101 and 103oC). Consequently, the rate of nutrient degradation in the peripheral region is higher than in the center (11).
The rate of heat transfer is influenced by the composition of the food and its properties of heat and mass transfer, including thermal conductivity, thermal diffusivity, specific heat and density. These characteristics change during the frying process, once oil and food are altered. Besides, there are other changes caused by interactions between food compounds (1).
Frying can be accomplished in batch or continuous fryers. Batch fryers are typically smaller and primarily used in catering service. Continuous fryers, which are capable of handling large amounts of frying oil and foods, are primarily used in industrial settings, and involve large-scale production. Fryers can be operated under atmospheric, high or low pressure, and even under vacuum (12). However, most large-scale production is done under atmospheric conditions.
The conditions to which food are submitted during the frying process initiate physical and chemical changes that depend on the composition of the food, and affect the development of color, flavor, and taste, besides changing food texture.
Table 1 summarizes the physical and chemical changes in food during the frying process. A portion of frying oil and polar compounds produced by oil degradation are absorbed by food, contributing to the quality of the final product. An amount of water evaporates due to the dehydration that occurs during frying process. Furthermore, starches gelatinize, proteins denature, some nutrients are lost (such as the vitamins thiamin and riboflavin, which are unstable at high temperatures), flavors develop, crispness is produced and pores are formed, leading to distinctive texture and sensory characteristics (1).
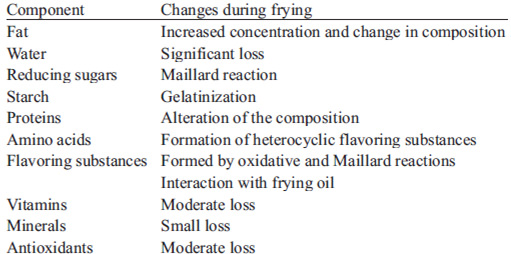
The content of carbohydrates and protein in raw material, strongly interacts with lipids, especially with thermal and oxidative degradation products (Maillard reaction products, reticulated proteins), generating toxigenic and carcinogenic compounds (13, 14).
Changes in color, taste, and flavor caused by Maillard reactions and caramelization
Changes in the food surface may be caused by caramelization and / or Maillard reaction (both responsible for the development of gold to brown hues), as well as evaporation of surface water, which characterizes crust formation, responsible for the texture of fried food (15). Color development in potato chips, for example, is proportional to the amount of reducing sugars in the potato, since both browning and Maillard reactions are stimulated by the level of oxidation of the food, and also by characteristics of heme pigments (15).
Maillard (non-enzymatic browning) is considered the most important reaction in the browning of food (16). During frying, this is the main reaction affecting sugars which involves free amino groups of amino acids, peptides, proteins, and carbonyl groups or other aldehydes, and ketones of sugars. Several intermediate products, called Amadori products or pre-melanoidins, are rapidly polymerized at frying temperatures, forming dark-colored molecules (melanoidins). Browning is faster at temperatures above 150 °C (17).
Among all the compounds produced by Maillard reaction, there is an increasing interesting in toxic compounds, like acrylamide. Acrylamide has been known to be a neurotoxic, genotoxic and carcinogenic compound in animal, and is classified as a probable human carcinogen (18). The acrylamide formation has not been fully elucidated, but it is established that it is formed via the Maillard reaction when asparagine and reducing sugars are heated at high temperature (19, 20). In fact, in one recent study, Miao et al. (21) showed that with the increasing of treatment time and temperature in potato chip, acrylamide contents were increased accordingly. Water activity could also influence the formation of this compound, with the decrease of water activity, the formation of acrylamide increased (21).
Besides Maillard and caramelization, frying oil can also take part in the non-enzymatic browning process by reaction of lipid oxidation products with amines, amino acids and proteins (22; 23).
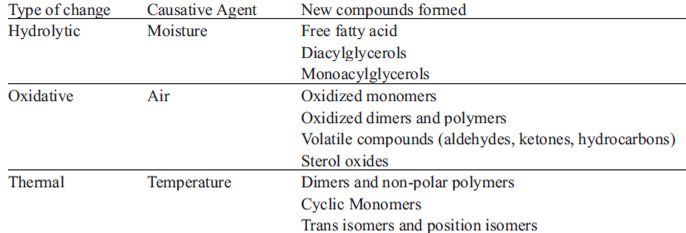
When food is immersed in hot oil in the presence of O2, the oil is exposed to three agents that cause changes in its composition: water from the food (which causes hydrolytic changes), oxygen (that gets in contact with the oil and causes oxidative changes from the surface to inside of the food) and finally, the high temperature (which causes thermal changes, such as isomerization and scission reactions - aldehydes and ketones - forming various degradation products, as epoxides and hydroperoxides (15,24).
The characteristics of color, taste and flavor of fried products are also developed by a combination of reactions and compounds absorbed by the frying oil. The main factors affecting the changes in color and flavor during the process are the oil type, storage and thermal changes, interfacial tension between the oil and the product, temperature and length of frying, moisture content, size and characteristics of food surface and pre-frying treatments (4).
Lipid oxidation generates volatile and non-volatile compounds that interfere in taste and flavor. The volatiles in the frying oil increase at the beginning of the process, but decrease during the frying. There are volatiles important to the quality of the process, such as saturated aldehydes C6-C9, enals (e.g., 2-decenal), dienals (e.g., 2,4-heptadienal), and hydrocarbons (hexene, hexane, heptane, octane, nonane, and decane). The formation of nonvolatile decomposition products is due to the oxidation and polymerization of unsaturated fat acid. Aldehydes affect the flavor of deep-fried foods, as 2-Trans-4-trans-decadienal that contributes to a flavor while other aldehydes produce off flavor (25).
Thermal stability of oils depends on their chemical structure. According to Reda (15), saturated oils are more stable than unsaturated ones. Unsaturated fatty acids are the main precursors of the volatile compounds found in oxidized oils (26,27). Linolenic acid is an unsaturated fatty acid essential to health that is rapidly lost in the frying process, altering the balance between saturated and unsaturated fatty acids in the oil (4), and increasing off-flavor formation (28).
The uses of the oil during a long time and/or its reuse lead to formation and accumulation of undesirable substances. These compounds may be related to the release and dissolution of food particles, or products of thermal and oxidative reactions in the oil. All these factors contribute to increase oil viscosity, decrease surface tension between the food and the oil, and increase the supply of oil on the food surface, facilitating oil absorption (29, 30).
Incorporation or absorption of oil depends on the initial quality and type of oil or fat used (31). According to Paul and Mittal (32), many factors affect the penetration of oil into the food, such as the geometric shape, oil viscosity, food type, oil temperature, and length of frying.
According to Fellows (4), development of texture inside the food during the frying process is a result of the combination of changes in proteins, fats and carbohydrate polymers similar to those that occur during boiling or baking.
The development of pores is a major structural change. Pores are formed by evaporation of water and formation of capillaries. Intense heat leads to explosion during evaporation of water, creating wide pores. A superficial crust is quickly formed, acting as a barrier to evaporation, decreasing the loss of water and keeping the inside of the food moist. Starch gelatinization and protein denaturation also contribute to the development of pores and shrinkage of food because gelatinized starch is usually dispersed in the continuous phase formed by protein denaturation. According to Ngadi and Xue (1), the presence of pores affects mechanical properties of the food and, consequently, its texture and acceptability.
The structure of a protein is the result of various intermolecular attraction and repulsion interactions, such as the interaction of protein groups with water. Changes in natural structural conditions of the protein can be result of changes in temperature, pH, surface tension, presence of salt, and other agents that disrupt the intraand intermolecular interactions, causing changes in the structure and breaking the protein in amino acids chains. These amino acids can form spherical aggregates which interact and originate the gel network (33).
Some lipids present in fried food are oxidized, may be accelerated by temperature increasing and oxygen concentration. Although nutritional effects are difficult to be estimated due to the variety of interfering factors, such as the type of oil, history of thermal treatment, and portion retained in the food (4). Due to oil incorporation, it is important that the choice of the frying oil is based not only on its technological characteristics, but also on its nutritional features (34).
The increase in energy intake is one of the principal problems associate with consume of frying foods. The fat content of food increases due to absorption and retention of oil, which implies an increase in energy intake on average in 42% in French fries and 53% in hamburger (35). The Dietary Guidelines for Americans recommend that less than 35% of daily calories be contributed from fat, but in fried foods, up to 75% of calories can be from fat (36).
Digestibility of fat is also changed when the process is accomplished using reused oils / fats. Even if a frying oil regulation is established, limiting polar compounds to 25 % and polymer content to 12 %, potentially toxic compounds can appear in oil (2).
The hazardous compounds presently identified are potentially carcinogenic molecules, such as carbonyl compounds or monoepoxides, and some aldehydes produced from linoleic acid. For example, 4-hydroxy-2-transnonenal has been proven to be cytotoxic (2, 37).
Frying process also can produce trans fatty acids (TFAs). TFAs are defined as unsaturated fatty acids that contain non-conjugated carbon–carbon double bounds in the trans configuration and epidemiologic studies suggested that there is a relationship between the level of these compounds intake and the risk of cardiovascular disease (38, 39).
TFAs formation in the oil during frying have been investigated (39, 40, 41). According to these studies, degrees of TFAs formation during frying depended on three main factors: frying condition, frying materials and the methods of TFAs measurements (39).
Nutritive value of food proteins is a combination of quality and quantity. Quality represents functional content of the protein consumed and used by the organism. Quantity represents the protein content in the food. Heat treatment can reduce the amount of protein and destroy some amino acids, changing the quality of protein composition in food (42).
Nevertheless, the protein content increased after frying in grass carp fillet (43). In another study, proteins of the fried sardine were higher than those of the untreated ones. This may be due to the formation of new products similar to protein during the frying processes and could have influenced the determination of protein content using Kjeldahl method (44, 45).
The frying process reduced the amino acid contents, with the lowest value was obtained in samples fried in palm oil (46). Deep-fat frying had been reported to decrease the available lysine of fish fillets by about 17% and by 25% when the fish oil had been used for continuous frying for 48 h as a result of interactions between the amino group of lysine and carbonyl compounds (46).
Netherless, no significant effect of the frying practices was found on the amino acid content (lysine, histidine, threonine, valine, methionine, leucine, isoleucine, phenylalanine, arginine, aspartic acid, serine, glutamic acid, proline, glycine, alanine, cystein and tyrosine) of selected fishes (45). A study on the amino acid contents of raw, cooked and fried fishes no found differences in individual amino acids as result of frying (47).
In general, the content of protein is increased by the frying process due the effect of concentration, because frying is also a process of dehydration. In relation of essential aminoacids, there is no agreement in the literature about it loss. However, lysine is the first aminoacid involved in Maillard reaction, so it is supposed to be lost during frying process.
The content of minerals seems to have no significant loss. Studies found that minerals are relatively preserved by frying, especially at high temperatures (165 to 185 oC), and short cooking time (48, 49). Özeren and Ersoy (50) evaluated fish processing and observed a small increase in the concentration of minerals such as Na, K, Ca, Mg, Fe and Zn after frying, probably due to a concentration effect. In a study with rainbow trout fillets the minerals (Na, Ca, Mg, K, P, Fe and Zn) increased significantly during the frying process (51).
Another study determined the effects on the content of minerals and heavy metals of frying frozen seafood. The process of frying increase significantly the content of macro and microelements as well as the content of heavy metals (Na, Mg, Ca, Cu, Fe, Zn, Pb, Cd and Hg ), except for K and Mg content in mussels and shrimps, respectively (52). How was related to protein, the content of some minerals can increase with frying due the effect of concentration.
Vitamins are thermosensitive and their oxidation depends on the internal temperature of the food and frying process. Vitamin C usually is the most thermosensitive. Among B group vitamins, thiamine, riboflavin, niacin and B6 are the most frequently affected by the process (15).
Biswas and Nanni (53) reported moderate loss of vitamin C when frying fruits in soybean oil. Loss of vitamins is caused by high temperatures or enzymatic oxidation during the preparation process or long periods of frying (54). Speek et al. (55) investigated the effect of processing on carotenoids in vegetables, indicating an average loss of 14 % and 24 % for vitamin A activity in boiling and frying, respectively.
Manorama and Rukmini (56) investigated the effects of processing on the preservation of beta-carotene in palm oil. Total beta-carotene was eliminated in the first frying operation, but the mixture of other types of carotenes, even with some losses, was still found in the oil. Some carotene oxidation products were identified in heated palm oil, indicating that the loss of beta-carotene during frying is probably partly due to the oxidation of the compound (57,58).
Retinol, carotenoids and tocopherols are destroyed, changing oil flavor and color. However, preferential oxidation of tocopherols has a protective (antioxidant) effect which is particularly important, since the majority of the frying oils is of vegetable origin, showing great amounts of unsaturated, rapidly oxidized fats (4). In oils with different unsaturation degrees, degradation was significantly higher when tocopherols were absent, can to be related with tocopherols type (59). Kourimska and Gordon (60) reported that the content of α-tocopherol was lost more quickly than other tocopherols of canola oil, with a 50 % reduction after 4-5 frying operations.
The influence of home cooking methods, such as frying on the antioxidant activity of vegetables was evaluated. Garlic showed losses higher than 50%, asparagus between 30% and 40%, Swiss chard, cauliflower, and pepper between 5% and 30% considering ABTS radical scavenging capacity (53).
The most common polysaccharide in food is starch. Amylose and amylopectin dissolve in water with heat forming a polymer network, leading to starch gelatinization (33). Gelatinization occurs after denaturation of globular proteins at high temperatures and involves carbohydrates, proteins, lipids and water (33).
When raw potato products are fried, changes in starch are very important. Starch granules are rapidly gelatinized upon contact with hot oil. The rigid structure of raw potatoes is lost in 1–2 min, and the fried chips become soft. On further heating, a firm crispy crust is formed on the surface of fried particles, which is highly appreciated by the consumer. On the surface of chips, where the water content is much lower than in internal layers, the gelatinization is not so intense, so starch granules partially retain their crystalline structure (61).
Studies about the dietary fiber content indicated that cooking of frozen French fries had no effect on starch composition compared with fresh samples, while frying significantly increased the percentage of resistant starch, partially attributed to the formation of amylose-lipid complex (62), increasing fiber content.
High-molecular-weight non-starch polysaccharides (dietary fiber) decompose during the preparation of French fries, especially in the case of low-specific gravity tubers. However in some cases dietary fiber increased during frying, possibly caused by the formation of melanoidins or other indigestible compounds. Besides, polysaccharides form a compact film on the surface at the beginning of the heating process, preventing fat migration into the fried food and loss of water (61, 63).
The degree of unsaturation of the fatty acids is the main factor affecting the oxidative stability of oil/fat. In general, oils that are more unsaturated oxidize more rapidly than less unsaturated ones. Minor components such as metals, free fatty acids, mono- and diacylglycerols can negatively affect frying stability of oil (65).
Reused oil gives better characteristics to the fried product, compared with oil that did not undergo temperature changes, because of the polar compounds that impregnate the food surface, providing flavor, improving contact between the oil and water on the surface of the product, and catalyzing heat transfer (66). However, the quality of the oil is affected when it is used for long periods. In continuous commercial production, oil is retained in the product and should be continuously replaced, and non-volatile products formed should be removed by filtration in order to maintain the oil quality (4).
Free fatty acids, mono- and diglycerides are hydrolysis products and represent about 2.5 to 4 % of the acidity of disposable oil. Its oxidation and reactivity rate is generally higher than triacylglycerols, making it easier for changes to take place. Free acidity in oil is a measure commonly used to control the quality of frying oils. Free fatty acids are also negative factors because they lower the smoke point (4).
Depending on the amount of water that the frying process removes from the food and that is mixed with triacylglycerols (TAG), these compounds may be hydrolyzed and form free fatty acids (FFA), monoacylglycerols (MAG) and diacylglycerols (DAG) and/or be oxidized by incorporation of oxygen from the surface, forming peroxides, hydroperoxides, conjugated dienes, epoxides, hydroxides and ketones. These compounds may be decomposed in small fragments or remain in the TAG molecule and bind with each other, leading to TAG dimers, polymers (5, 31).
The interactions between frying oil and fried food are of great relevance for nutritional quality of the final product. Foods are complex and heterogeneous matrixes, and all changes produced by frying occur simultaneously and contribute to the development of color, taste, texture and quality of fried products. Depending on the composition of the food, there may be a predominance and/or intensification of a particular reaction, which is difficult to delineate and analyze separately. The frying process can cause changes in the structure of labile nutrients, such as proteins, vitamins and antioxidants. Some compounds produced during frying process such as trans-fat acid and acrylamide are a public health problem. It is therefore very important to identify and evaluate their effects on human health and also how to reduce the production of these compounds during frying.
Recibido: 26-02-2013
Aceptado: 13-06-2013