In the present study a simple and highly sensitive RP-HPLC method has been established for simultaneous determination of chlorogenic acid, caffeic acid, vanillic acid and caffeine in coffee samples. The method has been applied to eight different coffees available on the Romanian market which were previously analysed concerning the total polyphenols content and antioxidant capacity. Reduction of the DPPH radical was used to determine the antioxidant capacity of the coffee extracts while the total polyphenols content was determined by spectrophotometry (Folin Ciocalteu's method). The total polyphenols content ranged from 1.98 g GAE/100 g to 4.19 g GAE/100 g while the caffeine content ranged from 1.89 g/100 g to 3.05 g/100 g. A large variability was observed in chlorogenic acid content of the investigated coffee samples which ranged between 0.6 and 2.32 g/100 g.
Key words: Phenolic acids, caffeine, HPLC, antioxidant capacity, coffee
En este trabajo, un método sensible RPHPLC fue desarrollado para la determinación simultánea del ácido clorogénico, del ácido cafeico, del ácido vanílico y de la cafeína en las pruebas de café. El método fue aplicado para analizar ocho tipos de café disponibles en el mercado de Rumania, que fueron analizados también en cuanto al contenido total de polifenoles y la capacidad antioxidante. La reducción del radical DPPH fue utilizada para determinar la capacidad antioxidante de los extractos de café mientras que el contenido total de polifenoles fue determinado por la espectrofotometría (método Folin Ciocalteu). El contenido total de polifenoles se situó entre 1,98 g GAE/100 g y 4.19 g GAE/100 g mientras que el contenido de cafeína se situó entre 1.89 g/100 g y 3.05 g/100 g. Una gran variabilidad se observó con respecto al contenido de ácido clorogénico en las pruebas de café analizadas, contenido que se situó entre 0.6 y 2.32 g/100 g.
Palabras clave: Ácidos fenólicos, cafeína, HPLC, capacidad antioxidante, café
Department of Chemistry, Sciences Faculty, University of Craiova, Craiova, Romania;
Department of Horticulture & Food Science, Agriculture & Horticulture Faculty, University of Craiova, Craiova, Dolj, Romania
Coffee has been one of the most popular beverages all over the world, and its consumption continues to increase (1). An interesting characteristic of coffee brews is that they have been consumed mainly for pleasure, without concern about the nutritional value (2).
Coffee is a well-known and extensively utilized psychotropic agent with effects on mood, cognitive performance, and motor activity. In fact, many investigators have shown that caffeine, one of the main constituents of coffee, has a variety of pharmacological and cellular responses in a wide spectrum of biological systems. These include stimulation of the central nervous system and cardiac muscle, increased urinary output, and relaxation of smooth muscle (3). Research has suggested that caffeine can be a potential contributor to reducing risk factors involved in the metabolic syndrome, including type 2 diabetes mellitus and obesity, and symptoms associated with Parkinson’s disease (4). However, its stimulatory effects may also adversely affect sensitive individuals by causing tachycardia, increase of blood pressure, anxiety, and insomnia (5).
Besides its stimulant effect, coffee has properties to prevent the deleterious actions of free radicals and viral infections (6). In various biological tests, the water extract of coffee showed superoxide anion-scavenging effects, inhibitory activity of lipid peroxidation, and suppression of hepatitis B virus surface antigen. These biological activities are closely related to the presence of caffeic acid derivatives, especially chlorogenic acids (7). Coffee is the major source of chlorogenic acid in the human diet. On the basis of 10 g of coffee per cup of brew, a cup contains 15−325 mg of chlorogenic acids; daily intake of coffee drinkers is 0.5−1.0 g, whereas coffee abstainers typically ingest < 100 mg/day (8, 9). Few free phenolic acids are present in coffee, although small quantities of caffeic, ferulic, and vanillic acids have been detected (10), the amount of the latter compound increasing in the medium-and dark-roasted samples (9). Data have been published regarding the ability of chlorogenic acids metabolites, such as ferulic, isoferulic, or vanillic acids, to exert radical scavenging activity (10, 11).
The concentration of highly active polyphenols in green coffee beans is influenced by the species and its origin while in coffee beverages it depends on the brewing procedure. During roasting phenolic compounds are partially degraded and/or bound to polymer structures depending on roasting conditions while other antioxidant compounds, such as Maillard and Strecker reaction products, are developed enhancing overall antioxidant properties (12 - 14). A positive but nonlinear relationship was found for the amount of chlorogenic acids that remained after roasting and antioxidant activity of beans. Multiple studies suggest that melanoidins are responsible for the strong antioxidant properties exhibited by roasted coffee beverages (15).
Various analytical methods have been used for the determination of phenolic compounds (chlorogenic acid and its derivatives) and caffeine in coffee beans and other plants. The most widely used methods are HPLC (2, 5, 8, 15, 16) but UV-VIS spectroscopy (17), gas chromatography after derivatization (18), capillary electrophoresis (19) and micellar electrokinetic chromatography (20) were also used.
In this contribution a simple RP-HPLC method was developed and validated for the simultaneous determination of chlorogenic acid, caffeic acid, vanillic acid and caffeine in commercial coffee samples. The method was applied to quantify these compounds in various commercial coffees. Also, we report the antioxidant activity and total polyphenols content of these coffee samples. The purpose was to provide simple methods in order to evaluate some important characteristics which define the quality of the commercial coffees entering the Romanian market and to establish the dietary intake of bioactive compounds in these products.
A total of eight different brands of coffee were considered for inclusion in our study: five roasted ground coffee samples, vacuum packaged, and three roasted coffee beans samples. Three batches of each brand were purchased in commercially available size from local supermarkets and composite samples were prepared for analysis.
For the determination of the total phenolic content, the Folin-Ciocalteu reagent (2 N, Merck), gallic acid (99% purity, Sigma), anhydrous sodium carbonate (99% purity, Sigma) were used. For the determination of the antioxidant activity, DPPH (1,1-diphenyl-2-picrylhydrazyl) (Sigma-Aldrich), ascorbic acid and methanol (Merck) were employed.
For the HPLC analysis, chlorogenic acid (>95%), caffeic acid (>98%), vanillic acid (>97%), and caffeine (>99%) standards were purchased from Sigma-Aldrich. HPLC-grade methanol, ortophosphoric acid and acetonitrile were from Merck. The water used was ultrapure, SG-Water system.
Coffee beans were ground to a powder. Five grams coffee powder were mixed with 50 mL of water at 100oC and left for 15 min. The resulting coffee brews were filtered. Filtrates were tightly closed and stored at 4oC prior to analysis. For all the analysis samples were run in triplicate.
The total polyphenols content was determined by spectrophotometry (UV-VIS Evolution 600) according to the Folin-Ciocalteu’s method. Briefly, 1 mL of diluted extract (1:20) was transferred in duplicate to separate tubes containing 1 mL ultrapure water and then 5 mL of Folin-Ciocalteu’ reagent (diluted 1:10) were added. After 2 min, 4 mL of sodium carbonate solution (7.5% w/v) were added. The tubes were then allowed to stand at room temperature for 120 min before absorbance at 765 nm was measured against ultrapure water. The total polyphenols content was expressed as g of gallic acid equivalents (GAE)/100 g material. The concentrations of polyphenols in samples were derived from a standard curve of gallic acid ranging from 50 to 250 mg/mL.
The antioxidant activity of coffee extracts was measured in terms of hydrogen-donating or radical scavenging ability, using the stable radical DPPH. 2.95 mL of methanolic solution of DPPH (0.004%, w/v) were added to 50 μL of extract and shaken vigorously. The tubes were allowed to stand in darkness at 20oC for 30 min. The decrease in absorbance at 517 nm was determined after 30 min in an Evolution UV-VIS 600 spectrophotometer (zeroed on pure methanol). Radical scavenging activity was expressed as the inhibition percentage and was calculated using the following formula:
Radical scavenging activity (%) = [1 – As/A0] × 100, where As is the absorbance of the sample (i.e., extracts) and A0 is the absorbance of the DPPH solution
HPLC was performed by using a Surveyor Thermo Electron system including vacuum degasser, Surveyor Plus LCPMPP pump, Surveyor Plus ASP autosampler, PDA5P diode array detector with 5 cm flow cell and Chrom Quest 4.2 system manager as data processor. Separation was achieved by a reversed-phase DS Hypersil C18 column (5 μm particle size, 250 mm×4.6 mm). A mobile phase composed of 0.2% ortophosphoric acid/acetonitrile (90:10) in isocratic conditions was used throughout the analysis. The samples were eluted for 16 min at a constant flow rate of 1.0 mL/min and the injection volume was 5 μL. The column temperature was 20°C and UV detection was carried out at 254 nm for vanillic acid, 275 nm for caffeine and 323 nm for chlorogenic and caffeic acids. Identification was based on the comparison of the spectra obtained between 200-500 nm and the retention time of the unknown substances in relation to that of pure standards.
The mobile phase was filtered through a polyamide membrane (0.2 μm) and degassed with an ultrasonic bath DK 102p Bandelin before use. Quantification was achieved by external calibration, using a five-point curve of different dilutions of a standard solution. Calibration curves were constructed for the compounds evaluated at five concentrations ranging from 20 to 120 mg/L for chlorogenic acid and caffeine and from 1.5 to 7.5 mg/L for caffeic and vanillic acids. Each calibration point was the mean of three independent measurements. The coffee extracts were filtered through nylon syringe filter (0.45 μm) before injection and were analyzed with no other modification than the appropriate dilution to fit the standard curves.
Data were expressed as means ± SD of three independent experiments carried out in triplicate. Data were evaluated by one-way analysis of variance (ANOVA) using Statgraphics Centurion XVI software (StatPoint Technologies, Warrenton, VA, USA). Differences in content levels among the coffee samples were estimated with a multiple range test using the least significant difference (LSD) at P < 0.05.
Simultaneous determination of chlorogenic acid, caffeic acid, vanillic acid and caffeine in coffee samples
The analytical curves for chlorogenic acid, caffeic acid, vanillic acid and caffeine were established from the HPLC output for standard solutions. Each standard solution was injected in triplicate into the HPLC system and the calibration curves were established by plotting peak areas against the corresponding concentrations for each compound. The retention times, regression equations and linear determination coefficients are shown in Table 1. The linearity of calibration curves for all compounds was very good (r2 > 0.999).
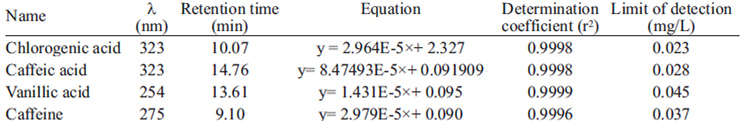
Peak areas were checked for repeatability at two concentration levels by injecting the mixed standards solutions into the HPLC system and calculating the relative standard deviation (RSD) for 10 replicate determinations. The repeatabilities of the retention times were below 0.5% while the repeatabilities of peak areas were all below 2%.
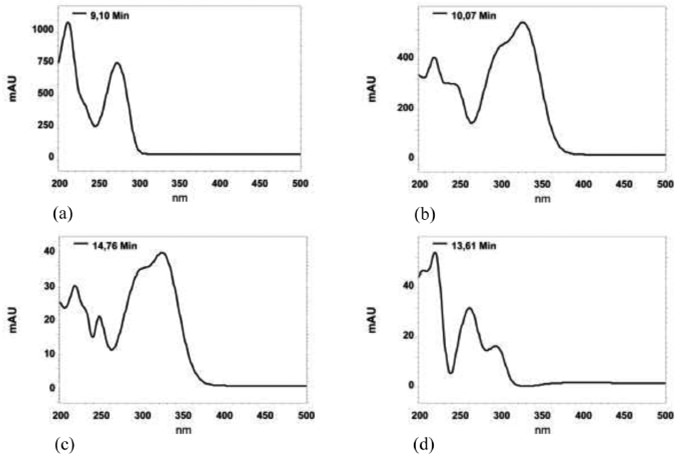
The use of a diode array detector allowed us to confirm the identity of the peak not only by its retention time, but also by the overlay of the UV-VIS spectra with a standard. Figure 1 illustrates the UV-VIS absorption spectra of caffeine, chlorogenic acid, caffeic acid and vanillic acid respectively, measured in methanol in the wavelength range of 200 - 500 nm.
The limits of detection (LODs) of the individual compounds were calculated at their absorbance maxima on the basis of a signal-to-noise ratio of 3 (table 1).
Recovery and repeatability experiments were conducted to evaluate the precision and accuracy of the method. The precision of the method was confirmed by repetitive analyses, calculating the average relative standard deviation (RSD) for 8 replicate determinations. This analysis was repeated over three days. For retention time, RSD values were between 0.065% and 0.233% while for peak areas RSD values ranged between 0.165% and 0.423%.
The recovery was evaluated by adding known amount of a standard solution to a coffee brew sample and by performing six replicates of the sample before and after fortification. The recovery values were 98.2 ± 0.32% for chlorogenic acid, 100.4 ± 0.16% for caffeic acid, 96.6 ± 0.38% for vanillic acid and 99.8 ± 0.23% for caffeine. RSD of the results for coffee samples were within acceptable limit (RSD < 2%), proving a good precision of the method while the recoveries ranged from 98.2% to 100.4%, proving a good accuracy.
The total polyphenols content of coffee samples investigated in this study varied from 1.98 g/100 g to 4.19 g/100 g (Table 2). Nebesny and Budryn (1) found total polyphenols contents between 2.06 and 3.03 g/100 g in green coffee beans roasted under different conditions. Also, in a study about Indian monsooned coffee (21), the total phenolic content was found to be 3.6 g/100 g in Monsooned Malabar coffee and 4.0 g/100 g in Monsooned Robusta coffee in terms of gallic acid equivalents.
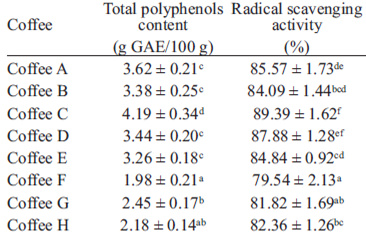
Reduction of the DPPH radical, which is an acceptor of hydrogen atoms from antioxidants, and is thus converted to DPPH-H, results in a decline in molar absorbency coefficient at 517 nm and a simultaneous change in the color of the solution from purple to yellow. The DPPH radical scavenger activities of the aqueous extracts of coffee samples are presented in table 9.
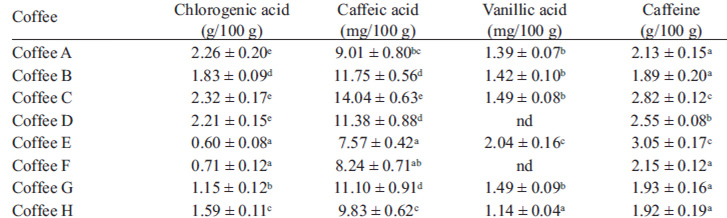
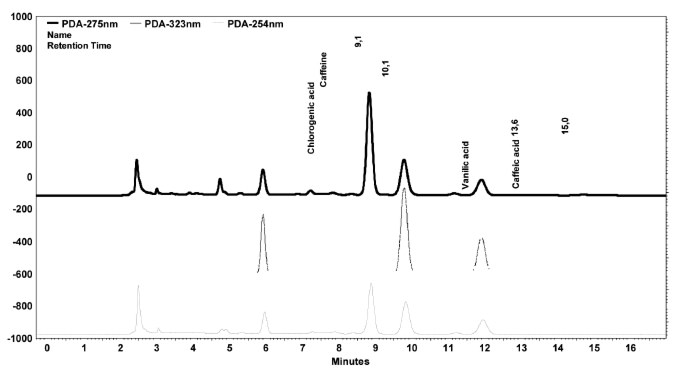
Content of chlorogenic acid, caffeic acid, vanillic acid and caffeine in the aqueous extracts of coffee samplesFrom the equations in Table 1 and chromatogram peak areas, the concentrations of chlorogenic acid, caffeic acid, vanillic acid and caffeine of coffee samples were established (Table 3). Typical chromatograms at λ=254 nm, λ=275 nm and λ=323 nm are shown in Figure 2.
Roasting is one of the most important factors influencing the phenolic content. Roasted beans appeared to contain less polyphenols than the green ones, since more than 60% of chlorogenic acid present in green coffee is degraded upon roasting (1). Sacchetti et al. (14) found that the concentration of total phenolics in brews from different types of coffee decreased linearly with increasing intensity of roasting thermal process. Also, the initial humidity of green beans had a stronger impact on final polyphenol concentration in roasted beans, than the roasting method (1).
A good correlation (r2 = 0.85) was found between total phenolics content of coffee extracts and their antioxidant activity. Previous studies also showed that the antioxidative effectiveness is, to the highest extent, correlated with polyphenols concentration (1). The antioxidant activity changes in brews from medium and dark roasted coffee are negatively influenced by the intensity of thermal process and seem to be much more dependent on roasting severity than on the type of coffee (14). Other factors which influence the free radical scavenging are the roasting method and the humidity of the beans before roasting (1).
Antioxidant activity of coffee samples was not correlated with chlorogenic or caffeic acid content, so other coffee compounds in addition to these phenolic acids are also responsible for the antioxidant activity associated with coffee consumption.
The caffeine content ranged from 1.89 g/100 g to 3.35 g/100 g. These values were consistent with published values. Thus, Nebesny and Budryn (1) found caffeine contents between 1.82 and 2.08 g/100 g in green coffee beans roasted under different conditions. Similar results were obtained by Daglia et al. (22), that detected 1.8–3.0% caffeine in green beans, 1.7–2.1% in medium roasted beans, and 1.6–1.9% in strongly roasted ones and by Farah et al. (5) that reported caffeine content in regular coffees around 2.54–3.33%.
Chlorogenic acids have an important role in determining coffee beans quality and beverage taste and are a key contributor to the antiperoxyl radical activity of coffee brews. Previous studies demonstrated that there is a loss of chlorogenic acids during roasting. The higher the roasting degree the lower is the content of chlorogenic acids (12). Budryn et al. (16) found that concentration of chlorogenic acids in dark roasted Robusta was 14-fold lesser than in green beans whereas in Arabica it was about fivefold lesser. Abebe Belay and Gholap (17) found 4.09-5.34%, 3.83-4.77% and 3.01-3.63% chlorogenic acids in coffee roasted at light, medium and dark temperature respectively, and they concluded that there is a significant decline in chlorogenic acids content as the roasting temperature increases. In our study, a large variability was observed in chlorogenic acid content of the coffee samples which ranged between 0.6 and 2.32 g/100 g, suggesting that these coffee beans were roasted under dark or very dark conditions. The richest coffees were those that registered also the highest total polyphenols content. The results were in good agreement with the previous reports about the antioxidants of coffee. Thus, Votavová et al. (8) found concentrations of chlorogenic acid between 0.4 and 2.8 g/100 g, while Farah et al. (13) found levels of 1.0-3.4 g/100 g in coffee samples with different roasting degree. Fujioka and Shibamoto (23) also found chlorogenic acids contents between 0.53 and 1.71 g/100 g in various commercial brewed coffees.
Caffeic acid is a bitter taste compound, which is usually found in small quantities in the processed arabic coffee. Caffeic acid elicits several interesting and various biological responses, such as antibacterial, anti-fungal, anti-inflammatory, antiviral, anticancer, antioxidant, antimutagenic, and anti-diabetic activities (24). Coffee samples evaluated in our study recorded caffeic acid content between 7.57 and 14.04 mg/100 g. These results are in agreement with those obtained by Chu et al. (19) who found 11.48 mg/100 g in a coffee sample using capillary electrophoresis with amperometric detection. Murthy and Manonmani (21) found higher levels of caffeic acid, specifically 20 mg/100 g in Monsooned Malabar coffee and 40 mg/100 g in Monsooned Robusta coffee. The increased levels of free caffeic acid was attributed to the hydrolysis of chlorogenic acid during the curing of this type of speciality coffee by exposing them to moist monsoon winds prevailing in the coastal regions of Mangalore and Tellichery.
Considering the results of vanillic acid content, the highest level was registered in coffee E (2.04 mg/100 g) which was significantly higher in comparison with the values determined in the other coffees. Vanillic acid was not detected in two coffee samples.
Coffee supplies a health-promoting mixture of phytochemicals such as natural antioxidants, which may contribute to the numerous health benefits associated with its consumption. The data presented in this work show the presence of phenolic compounds and caffeine in substantial quantities in coffee samples and provide evidence for the antioxidant potential of commercial coffees and their extracts. However, our results have shown the great variability in the bioactive components content of commercially available coffee brands. The concentration of these substances seems to vary considerably, since it depends on diverse factors, such as species and variety, the degree of maturation, and to some extent environmental conditions and agricultural practices. Processing, especially roasting, modifies dramatically the phenolic composition of coffee, producing aroma, flavor and color compounds characteristics of coffee beverage. The reversed-phase HPLC method developed and validated in this work for the simultaneous determination of chlorogenic acid, caffeic acid, vanillic acid and caffeine in coffee samples will contribute to the quality control of commercial coffees.
Recibido: 03-10-2012
Aceptado: 13-02-2013