This study aimed to evaluate iron (Fe) bioavailability in Wistar rats fed with rice fortified with micronized ferric pyrophosphate (FP) by Ultra Rice® (UR) technology with or without addition of yacon flour as a source of 7.5% of fructooligosaccharides (FOS). Diets were supplied with 12 mg iron/kg from the following sources: ferrous sulfate (FS - control diet), fortified rice with micronized ferric pyrophosphate (Ultra Rice®) (UR diet), ferrous sulfate + yacon flour (FS + Y diet) or Ultra Rice® + yacon flour (UR + Y diet). Blood samples were collected at the end of depletion and repletion stages for determination of hemoglobin concentration and calculation of the relative biological value (RBV). Also, the content of short chain fatty acids (SCFA) (acetic, propionic and butyric acids) from animals’ stools and caecum weight were determined. The UR diet showed high iron bioavailability (RBV = 84.7%). However, the addition of yacon flour in the diet containing fortified rice (UR + Y diet) decreased RBV (63.1%) significantly below the other three groups (p<0.05). Groups that received yacon flour showed higher acetic acid values compared to those who did not. In conclusion, fortified UR® with micronized ferric pyrophosphate showed high iron bioavailability but the addition of yacon flour at 7.5% FOS reduced iron bioavailability despite increased caecum weight and SCFA concentration.
Key words: Ultra Rice®, relative biological value; hemoglobin; iron deficiency; fructooligosaccharides.
Este estudio tuvo como objetivo evaluar la biodisponibilidad de hierro (Fe) en ratas Wistar alimentadas con arroz fortificado con pirofosfato férrico micronizado por medio de la tecnología Ultra Rice® (UR®), con o sin adición de harina de yacón. Las dietas contenían 12 mg de hierro/kg a partir de las siguientes fuentes: sulfato ferroso (SF - dieta de control), Ultra Rice® (dieta UR®), sulfato ferroso + harina de yacón (dieta SF + Y) o Ultra Rice® + harina de yacón (dieta UR® + Y). Al final del estudio, se recogieron muestras de sangre para la determinación de la concentración de hemoglobina y el cálculo del valor biológico relativo (RBV). También se determinó el contenido de ácidos grasos de cadena corta (AGCC) (ácidos acético, propiónico y butírico) en las heces de los animales. La dieta UR® mostró alta biodisponibilidad de hierro (RBV = 84,7%). Sin embargo, la adición de harina de yacón en la dieta que contenía arroz fortificado (dieta UR® + Y) disminuyó el RBV (63,1%) (p <0,05). Los grupos que recibieron harina de yacón mostraron los valores más altos de ácido acético en comparación con aquellos que no recibieron. En conclusión, el arroz fortificado con pirofosfato férrico micronizado por medio de la tecnología UR® mostró alta biodisponibilidad de hierro. La adición de harina de yacón, con el fin de proporcionar 7,5% de fructooligosacáridos (FOS) en la dieta, causó aumento del peso del ciego y de la concentración de AGCC, aunque disminuyó la biodisponibilidad de hierro.
Palabras clave: Ultra Rice®, valor biológico relativo, hemoglobina, deficiencia de hierro, fructooligosacáridos.
Department of Nutrition and Health, Federal University of Viçosa, Brazil. Department of Husbandry, Federal University of Espírito Santo, Center for Agricultural Sciences, Alegre, Brazil.
Iron deficiency is the most common and widespread nutritional disorder in the world, and is a public health problem in both industrialized and non-industrialized countries. Interestingly, although a marked decline of stunting and an increase in the obesity epidemic characteristic of the nutritional transition process have been observed, a high prevalence of anemia continues, with a modal frequency of 40-50% in children under five years and 30-40% in pregnant women. Anemia is, in terms magnitude, the main problem of deficiency in the world, apparently without major geographic differentiations (1).
Food fortification is a well recognized approach to overcome hidden hunger in many parts of the world, particularly in developing countries. Additionally, fortification is a method of controlling micronutrient deficiency as an intervention alternative mainly recommended for locations where high prevalence rates are found (2).
Several condiments and foods, including sugar, soybeans, milk, oil, wheat flour and rice have been explored as potential vehicles for fortification in different country contexts. Among these, rice is a potential candidate for fortification in countries where it is a staple food and specific deficiencies reach high prevalence rates among people with consistent rice consumption (3).
Previous attempts involving the fortification of rice flour were unsuccessful, due to the habit of washing and cooking rice with excess water, which results in the leaching of micronutrient used for enrichment (4). However, recently a new technology was created (Ultra Rice® - UR®) that overcame this barrier. Broken and cracked grains, which typically comprise 20% to 30% of the production and are generally destined for animal feed, can be transformed into rice flour, combined with a binder and other nutrients, and refurbished by extrusion as reconstituted rice grains with the same size, shape and texture of conventional rice (5).
Studies evaluating the efficacy and effectiveness of UR® in the improvement of the nutritional status of iron are still scarce. A study conducted in Indonesia demonstrated the viability of UR® in small rural mills and excellent market acceptance of the fortified product (6). In India, after seven months of using iron fortified-UR® in the meals of anemic schoolchildren, positive effects were observed with regards to serum ferritin (SF), but not hemoglobin (Hb) (7). In Brazil, it was found that micronized ferric pyrophosphate-UR® increased iron levels and reduced the incidence of anemia in children between 6 and 24 months old who were mildly anemic at baseline (8).
Iron bioavailability may be enhanced by dietary components, such as dietary inulin-type fructans (ITF) (inulin and fructooligosaccharides - FOS), as a result of their fermentation in the large intestine (9). Fermentation favors the production of short-chain fatty acids (SCFA), which affect luminal pH, in turn affecting mineral solubility. These effects are also accompanied by modifications in the mucosal architecture of the intestine as a result of increases in both the cellularity and number of crypts, mechanisms which may contribute to an increase in the mineral absorptive surface (9). However, in the study of Petry et al. (10) the authors found no effect of inulin on iron absorption. A possible explanation for this is that whereas it seems probable that iron is absorbed in the colon in human, colonic absorption is likely to be a minor component of total iron absorption compared with duodenal absorption
Yacon (Smallanthus sonchifolius) is an Andean tuberous root that accumulates large amounts of low degree of polymerization ITF (11). Its cultivation has expanded in various regions of the world (New Zealand, Japan, Czech Republic, South Korea, Thailand, Philippines, Russia, Estonia, Brazil etc..) because of its easy handling and processing and mainly because it is a source of bioactive components. Although it is considered a traditional food in South America, for the European Union yacon is a new food and therefore its safety must be evaluated. This has stimulated the interest of the scientific community in studies to characterize this root regarding to its chemical composition, technological and functional properties (12).
Yacon is regarded as a functional food given that it contains fructooligosaccharides (FOS), inulin and phenolic compounds. The consumption of FOS and inulin provides the growth of bifidobacteria in the colon, enhances mineral absorption and gastrointestinal metabolism (11). Therefore, the use of yacon, together with dietary sources of iron, may improve the mineral bioavailability and reduce the impact of dietary iron insufficiency.
In the study of Hunt JR (13), the bioavailability of some ferric pyrophosphate compounds in UR® was assessed. The authors found an relative biological value (RBV) varying from 75% to 94%. Therefore, although the ferric pyrophosphate bioavailability is good, the addition of yacon flour can improve it.
Thus, the present study aimed to determine whether the addition of yacon flour (Smallanthus sonchifolius) is able to increase iron bioavailability in Wistar rats fed with fortified rice with ferric pyrophosphate by UR® technology.
Rice grains extruded from rice flour (Ultra Rice® - UR®), produced by a pasta manufacturer (Adorella Foods Ltd.) located in Indaiatuba, Sao Paulo, Brazil, and kindly granted by Program for Appropriate Technology in Health (PATH) were used. The grains contained iron (in the form of micronized ferric pyrophosphate), zinc (as zinc oxide), thiamine (in the form of thiamine mononitrate) and folic acid.
Yacon roots (115.5 kg) were purchased from the local market (Viçosa, MG, Brazil), selected, weighted and subjected to the flour preparation process according to the methodology of Rodrigues, Castro, Martino & Ferreira (12). After washing in running tap water and sanitization in chlorinated water at 5 ppm during 5 minutes, peeling was made using a potato peeler and then roots were treated with a sodium citrate solution at 0.5% during 15 minutes. After grinding in multiprocessor (Walita, modelo RI7625), yacon pieces of 2 cm, whole peeled, was immersed in a sodium bissulfite solution at 0.5% during 15 minutes. After that, liquid was eliminated and drying was performed in airflow dryer (Polidryer-DP, Viçosa, Brazil) at 55ºC during 48 hours. At the end of the drying process, yacon was ground and the flour was weighted and stored in plastic bags at 10ºC. Chemical composition of yacon flour was determined as indicated by the AOAC method (14), resulting in the following values per 100 grams: 6.9 g of moisture, 2.7 g of proteins, 0.15 g of fat, 5.4 g of ash, 8.6 g of glucose, 21.1 g of fructose; 16.3 g of sucrose and 25.7 g of FOS.
Iron content in UR®, yacon flour and experimental diets was determined according to AOAC (14). One gram of each sample, in triplicate, was weighted in tubes and digested using 10 mL of concentrated HNO3 at a temperature of 160 °C. After the first 8 hours of digestion, another 5 mL of HNO3 were added. After completing digestion, the contents of the tube were transferred to 50 mL volumetric flasks. The samples were then mixed in a vortex and the volume was made up with deionized water. Iron was determined by plasma emission spectrophotometry (Perkin-Elmer Optima 3300 DV, Norwalk, USA). The glassware and utensils used for both the mineral and biological assays were demineralized, using a 10% HNO3 solution, in which they remained for 24 h followed by rinsing in deionized water.
The study was conducted according to Brazilian Standards of Animal Experimentation and was approved by the Ethics Committee for Animal Research (Project Identification Code: 33; date of approval: June 09th 2011).
Thirty-two 21-day old male Wistar rats (Rattus novergicus, albinus variety, Rodentia class), with initial body weight ranging from 60 - 90 g were used in the study. The animals were provided by the Central Biotery of the Universidade Federal de Viçosa, Minas Gerais, Brazil and were individually housed in stainless steel cages under controlled temperature (21°C ± 1°C) and 12 hour photoperiod for 14 days.
The depletion-repletion hemoglobin method was applied to determine iron bioavailability, according to AOAC (13), with a modification to the depletion phase which lasted three weeks, instead of four. This time was sufficient to cause iron deficiency anemia in the rats (6 mg/dL), based on the results of previous studies in our laboratory (15).
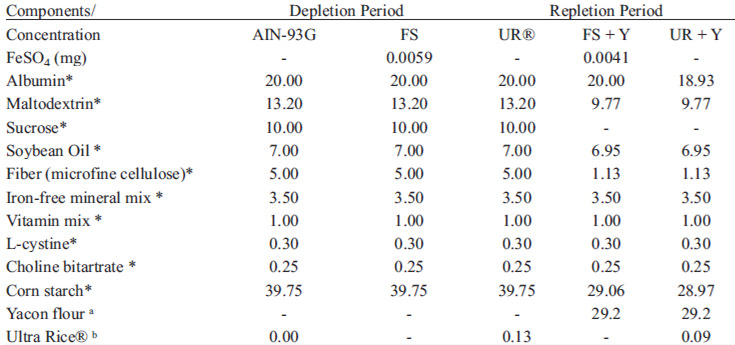
Diets were prepared based on the nutritional needs of animals using AIN93-G composition, according to Reeves, Nielsen & Fahey (16). The composition of the diets is presented in Table 1. Ingredients were individually weighed and mixed using demineralized plastic tools, followed by mixing in a semi-industrial mixer (Lieme®, São Paulo, Brazil) at low speed for 15 minutes. The diets were stored in polyethylene bags at 10ºC. The amount of yacon flour was calculated to provide 7.5% of dietary fructo-oligossacharides (FOS).
The animals were fed a modified AIN-93G diet (16) recommended for rats in the growing phase, utilizing an iron-free mineral mix and deionized water ad libitum, during 21 days to induce anemia. At the end of the depletion period, blood samples were collected by tail incision to determine hemoglobin (Hb) concentration. After making an incision at the terminal portion of the tail of each animal, blood was dripped on glass slide and immediately collected with a micropipette. A 10 μL blood aliquot was mixed with 2.5 mL of cyanide and potassium ferricyanide (Drabdkin solution) and absorbance was measured at 540 nm in a spectrophotometer (Shimadzu UV-1601).
Anemic rats were systematically assigned to four groups (n=8) according to their hemoglobin level to obtain groups with similar means. The groups were fed with diets containing 12 mg of iron/kg supplied from the following iron sources: ferrous sulfate (FS - control diet), fortified rice (Ultra Rice® - UR®) (UR® diet), ferrous sulfate + yacon flour (FS + Y diet) or Ultra Rice + yacon flour (UR® + Y diet). In diets FS + Y and UR® + Y, corn starch, sucrose and dietary fiber were quantitatively adjusted, taking into account the offer of 7.5% of FOS and the carbohydrate content of yacon flour (Table 1).
The rats were fed 17 to 18 g rations/day of repletion diet during 14 days and deionized water was provided ad libitum.
At the end of the repletion period, blood samples were collected by tail incision for further determination of Hb concentration.
For calculating Hb concentrations, absorbance for a standard Hb solution at a concentration corresponding to 11.4 g/dL was used as reference (Química Básica, Belo Horizonte, MG, Brazil).
Iron (Fe) consumption was calculated considering the total amount of diet consumed and the iron content of the specific diet, which was calculated for each animal according to the formula below:
The results of the Hb concentrations and iron consumption were used to estimate the following indexes:
During the experimental period, body weight and food intake were monitored to determine the feed efficiency ratio (FER), calculated as the ratio between the body weight gain (g) and food intake (g).
After the animals were sacrificed, a reticulocyte count was also obtained as an indicator of recent bone marrow activity. A 5 mL blood aliquot was collected in a tube containing EDTA, and then 0.5 mL of this solution was added to 0.5 mL of brilliant cresyl blue in a hemolysis tube, which was maintained in a water bath at 37 °C for 15 minutes. The smear was obtained from the homogenate which was focused using an objective lens. Counting was performed with at least 1.000 red blood cells, and the number of reticulocytes found in these fields was noted. Values were expressed in %.
For determination of SCFA (acetic, propionic and butyric acids) the method proposed by Smiricky-Tjarda, Grieshop, Flickinger, Bauer & Fahey (17) was used. SCFA concentrations were determined using high performance liquid chromatography system (HPLC) (Shimadzu, model SPD-10A VP), coupled to an Ultra Violet (UV) detector using a wavelength of 210 nm. After animals’ euthanasia, stools from the caecum were mixed with 25% metaphosphoric acid in eppendorf tubes and then maintained at rest for 30 minutes at room temperature. After that, samples were centrifuged in a refrigerated microcentrifuge (Hitachi, CT15RE) at 16.100 g for 30 minutes. The supernatant was transferred to another eppendorf and was centrifuged again for 20 minutes under the same conditions. This supernatant was then used for determining the SCFA concentrations. The chromatographic conditions were: reversed phase column (C18), 30 cm x 4.5 mm, flow rate: 0.8 mL/min, column pressure: 181 kgf, mobile phase: 1% orthophosphoric acid in water, injection volume: 20 μl.
Descriptive statistics were used and results are shown in terms of mean and standard deviation. Groups were compared using analysis of variance (ANOVA) and the Dunnett’s post hoc test was applied to identify where significant differences occurred, considering ferrous sulfate as the control group and a significance level of 5% (p <0.05). Data was analyzed using the software Statistical Package for the Social Sciences (SPSS), version 17.0.
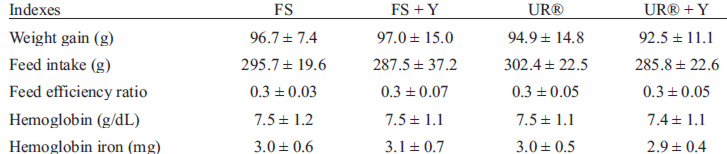
After 21 days of iron restriction (depletion period), it was observed that hemoglobin levels in the animals were low (Table 2), indicating the efficacy of iron depletion. No significant difference was observed in hemoglobin levels and hemoglobin iron among the experimental groups (p>0.05). Also, there were no significant differences among groups regarding weight gain and food consumption during the depletion period (p>0.05).
After analysis of diets, it was observed that the average ± SD iron content was 1.76 ± 0.23 mg iron/100 g in FS control diet, 2.11 ± 0.31 mg iron/100 g in FS + Y diet, 2.07 ± 0.29 mg iron/100 g in UR® diet and 2.53 ± 0.19 mg iron/100 g in UR® + Y diet.
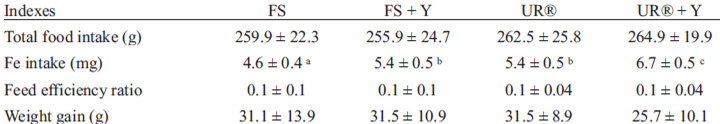
There were no significant differences (p>0.05) in food intake and FER among groups during the repletion period. However there was a difference in total iron intake (p<0.001), where the FS group presented the lowest intake (4.58 ± 0.39 mg) and the UR® + Y group showed the highest (6.71 ± 0.51 mg). Body weight and weight gain at the end of repletion period were not different among groups (Table 3).
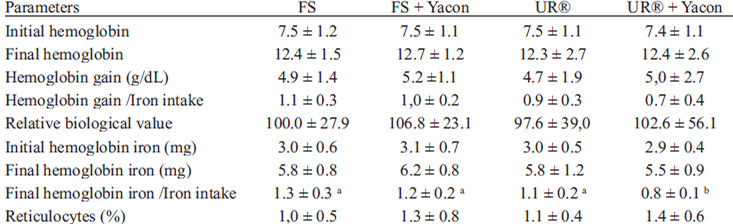
The hematological levels of animals consuming diets containing yacon flour and ferrous sulfate or UR® with ferric pyrophosphate as sources of iron at the beginning and end of the repletion period are described in Table 4 and Figure 1.
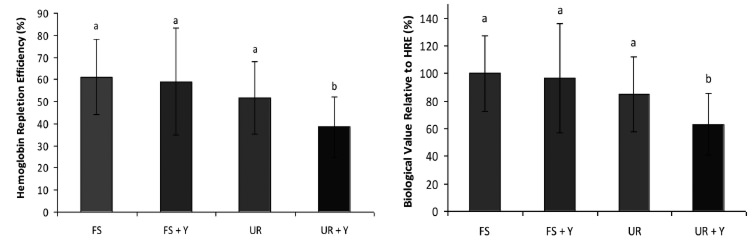
Iron intake was different among the groups since the iron concentration in diets were also different, although they were prepared to reach 12 ppm Fe/ kg of diet. These differences can occur due to possible contamination in ingredients used during the preparation of diets, which is common. Since significant differences were detected in iron intake among the groups, specific ratios were calculated in an attempt to avoid misinterpretation of the results since groups with higher iron intake have proportionally higher hemoglobin concentration and higher hemoglobin gain. Thus, the ratios hemoglobin iron/iron intake and Hb gain/iron intake were calculated.
Hemoglobin levels at the end of the repletion period were higher than in the beginning (end of depletion), however Hb gains did not differ among groups (p>0.05), even when this index was corrected by iron consumption.
Despite a tendency to increase in the groups that received yacon flour, the biological value relative to Hb gain did not differ among the groups receiving yacon compared with those that did not receive yacon (p>0.05).
Reticulocyte values at the end of the repletion period were normal in all groups. It was observed that at the end of the experiment the animals had recovered from iron depletion, with reticulocyte values within normal limits (0.5 to 2.3%).
There was no significant difference (p>0.05) in the levels of hemoglobin iron at the end of the repletion period; however, considering the iron intake, levels of hemoglobin iron were lower in the UR® + Y group compared to other groups.
Significant differences (p<0.05) were observed among groups with respect to hemoglobin repletion efficiency (HRE) and the biological value relative to HRE (RBV), as shown in Figure 1.
Yacon flour was associated with a significant increase in the absolute caecum weight of animals (p<0.001), more pronounced in the diet containing ferrous sulphate and yacon flour. The relative caecum weight (caecal weight/body weight) was higher in animals fed with yacon flour and more prominent in the group that received ferrous sulfate with yacon flour (p<0.001) (Table 5).
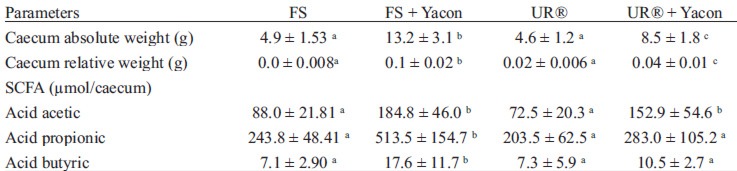
There was significant difference (p<0.001) in the concentration of SCFA, and with respect to acid acetic groups that received yacon flour showed higher values than those that did not. The FS + Y group produced higher butyrate and propionate concentrations, and no differences were observed in the other groups.
Iron bioavailability can be affected by sources of FOS. Lobo et al. (18) found that iron bioavailability from ferric pyrophosphate was higher when offering yacon flour to animals with iron deficiency anemia, with an RBV of 97%. It is important to consider that ferric pyrophosphate is an insoluble salt of low iron bioavailability. In the rat model, the high FOS content could improve bioavailability of this mineral by acidification of the medium resulting from the activity of fermentative local microbiota or increased iron uptake due to hypertrophy of the caecum walls (18). However, in the present study, rice fortified with ferric pyrophosphate showed a high RBV (84.7%) unlike that found by Lobo et al. (40%) (17). However, , when offered in association with yacon flour , the iron in the fortified rice showed a reduction in bioavailability. This indicates that in the present study pyrophosphate in fortified rice had the highest bioavailability and the addition of yacon flour reduced the bioavailability of this mineral.
One of the feasible reasons for the high iron bioavailability obtained in the present study may be that the iron pyrophosphate in UR was in the micronized form, unlike that used by Lobo et al. (18). According to PATH (19) and Wegmüller et al (20), the micronization process produces very small salt particles (micrometers), thus increasing the surface area and improving iron bioavailability when compared to larger particle-size ferric FP and other insoluble salts. Whereas the prebiotic effect at the hind gut level did not seem to increase iron absorption, the flour may have decrease iron absorption where it matters most – the upper small intestinal mucosa.
HRE values for the FS control group (61%) and for the FP UR® group (51.8%) were higher than those found in the study by Lobo et al. (18), in which FS showed an HRE around 25% and the FP group showed a HRE of approximately 10%. In the present study, despite having displayed the highest iron consumption among the groups (p<0.01), the group that received ferric pyrophosphate and yacon showed the lowest HRE and RBV (p<0.05).
One factor that may partially explain this result was the presence of a mild diarrhea observed in the group that received ferric pyrophosphate and yacon flour, which may have caused increased excretion of iron in the stool. However, the dose of FOS included in the diet was similar to that used in other studies, which did not report diarrhea in animals (9, 17).
Although negative effects of yacon flour on iron bioavailability in the present study were observed, there was a marked increase in caecal weight of the animals, being more pronounced in the group receiving yacon flour with ferrous sulfate. There was also an increased concentration of SCFA in these animals. Yacon flour contains a high proportion of carbohydrates in the form of oligofrutans such as inulin and FOS (21).
The effects of SCFA on both normal and neoplastic epithelial cells are known. While butyrate and, to a lesser extent, propionate, act by reducing the proliferation of tumor cells in vitro, all three major SCFA stimulate the proliferation of normal epithelial cells (22). It has also been reported that fermentation of FOS in the caecum of animals was accompanied by a hypertrophy in this portion of intestine or increase of the number and depth of caecal crypts (10), suggesting the cause/effect relationship between hyperplasia and hypertrophy and development of cell wall in the caecum.
Genta, Cabrera, Grau & Sanchez (23) presented results corroborating with those of the present study when feeding rats with diets containing yacon for a period of 4 months. Various levels of FOS (from 340 to 6800 mg/kg/day) were offered and the authors found significant increases in caecal weight only in the group supplemented with the highest level of FOS. These authors also found lack of toxicity and a certain beneficial metabolic activity in normal rats. In another study, Boyle et al. (24) fed rats with different oligofructose levels (0%, 0.55%, 1.65%, 4.96% and 9.91%), observing a significant increase in caecal weight for the supplemented diets. In these studies the increased caecum weight was dose dependent and was associated with the trophic effect of SCFA on colonocytes. In the present study 7.5% of FOS added to the diet increased caecal weight, especially when yacon was combined with ferrous sulfate. Significant gut effects of yacon flour were therefore observed, but not reflected in increased iron absorption.
Fortified rice with micronized ferric pyrophosphate by UR® technology showed high iron bioavailability. The addition of yacon flour, to provide 7.5% of fructooligosaccharides (FOS) in the diet, increased caecum weight and SCFA concentration, but reduced iron bioavailability, so its addition should not be encouraged.
Further studies are necessary regarding the effects of FOS, especially in diets with low bioavailability, aiming to reach further conclusions about its effects on iron absorption and contribution in the control of iron deficiency anemia.
Recibido: 13-12-2012
Aceptado: 13-06-2013