The present study aimed to determine the effects of different traditional cooking methods on folate (tetrahydrofolate - THF, 5-methyltetrahydrofolate - 5- MTHF and 5-formyltetrahydrofolate - 5-FTHF) retention in leafy vegetables. The analysis of folates was carried out by high performance liquid chromatography (HPLC), with detection by fluorescence, using gradient elution, mobile phase of acetonitrile and phosphate buffer solution. The retention of isomers in vegetables after cooking ranged from 17.0 % to 87.2 % for THF, 53.4 – 94.1% for 5-MTHF and 39.0 – 107.9% for 5-FTHF. The retention of folates depended on the food matrix, the kind of isomer, and the cooking methods used. It is recommended that one should have more control over the choices for methods and time of cooking and the amount of water used at home and at foodservice as well.
Key words: Cooking techniques, stability, tetrahydrofolate, 5-methyltetrahydrofolate, 5-formyltetrahydrofolate.
El presente estudio tuvo como objetivo determinar los efectos de los diferentes métodos de cocción tradicionales sobre la retención de folatos (tetrahidrofolato - THF, 5-metiltetrahidrofolato - 5- MTHF y 5-formiltetrahidrofolato - 5 FTHF) en hortalizas. El análisis de folatos se llevó a cabo por cromatografía líquida de alta resolución (CLAR), con detección por fluorescencia, usando elución en gradiente, fase móvil de acetonitrilo y solución tampón de fosfato. La retención de los isómeros en las hortalizas después de la cocción varió de 17,0% a 87,2% para THF, 53,4 a 94,1% para 5-MTHF y de 39,0 a 107,9% para 5- FTHF. La retención de folatos dependió de la matriz del alimento, el tipo de isómero, y los métodos de cocción utilizados. Se recomienda que uno debe tener más control sobre las opciones de métodos y tiempo de cocción y la cantidad de agua utilizada en el hogar y también en los servicio de alimentación.
Palabras clave: Técnicas de cocción, estabilidad, tetrahidrofolato, 5-metiltetrahidrofolato, 5-formiltetrahidrofolato.
Department of Pharmacy and Nutrition. Alto Universitário s/n, Guararema. Alegre-ES. Department of Nutrition and Health. Department of Food Technology. Universidade Federal de Viçosa-Viçosa, MG. Brazil.
Folates have received great attention due to their effect on health, especially on the reduction in the risks of neural tube defects, in the prevention of cardiovascular diseases and certain kinds of cancer (1).
Folates of natural occurrence are found in foods in a variety of ways. The most commonly detected in foods and leafy vegetables are the isomers 5-methyltetrahydrofolate (5-MTF), tetrahydrofolate (THF) and 5-formyltetrahydrofolate (5-FTHF) (2).
The folate synthesis is only performed by microorganisms and higher plants. Therefore, it is an essential nutrient for mammals (3). Vegetables are considered the main natural sources of folates in human diet (4).
The loss of folates may be caused by environmental factors, such as pH, oxygen, antioxidants, light, acids, alkalis, concentrations of metallic ions, cooking duration and methods, quantity of water used, besides the food’s own characteristics (5). Folate losses during the cooking process mainly occur by lixiviation, since folates have hydrosoluble characteristics, and by thermal degradation (6). Other factors, such as environmental conditions (season of the year, climate, and geographical and geological conditions), may affect the levels of folates in vegetables (4).
It is estimated that approximately 50% of the initial concentration of folates in foods is lost during the culinary processes (1). Some results reported that steam cooking and frying may cause losses of up to 90% in the initial concentration (7). According to the same author, vegetables may lose about 70% of their concentration of folates after being boiled for 8 minutes, and most of the losses occurs by dissolution in the boiling water. There are very few specific studies which evaluate the impact of different cooking methods on the concentration of folates in foods prepared both at home and at foodservices (1).
Among all the vitamins, folate belongs to one of the least studied compound groups, regarding the concentration in raw foods, as well as the stability throughout preparation. Particularly in Brazil, there is a great need for researches in this area, because there is no information available about the concentrations of folates in food composition tables. In fact, the methodology for the analysis of folates in foods requires complex procedures and standards poorly available in the world market.
The High Performance Liquid chromatography (HPLC) was used in the present work for the analysis of folates (THF, 5-MTHF and 5-FTHF) in vegetables highly consumed in Brazil (kale, spinach, mustard, florets and leaves of broccoli). Since these vegetables are offered almost all year long, the analysis of folates was also carried out in different seasons, considering the scarcity of information from previous works. Besides that, we compared the effects on folate stability of different cooking methods commonly used for vegetables in Brazil. The amounts of vegetables used in the preparations were based on those used in households.
Acetonitrile HPLC degree (Tedia, USA) and ultrapure water produced in Milli-Q® system (Millipore, USA) were used for the analysis of folates. Reagents with analytical grade used were: Anhydrous Monobasic Sodium Phosphate (Synth, Brazil), ascorbic acid (Vetec, Brazil), 2-mercaptoethanol (Vetec, Brazil), phosphoric acid (Proquímios, Brazil), sodium acetate (Chemco, Brazil) and sodium chloride (Vetec, Brazil).
A column was prepared to purify the extracts, with a stationary phase of Q-Sepharose (Pharmacia, USA) in 20% of ethanol 70%, using disposable plastic syringe for support, and flux established with peristaltic pump (Pharmacia Biotech).
The extracts were filtered with Inlab paper filter with a diameter of 9 cm. Before the analysis by HPLC, the extracts and standard solutions were filtered in HV Millex membrane filters, with 0.45 μm of porosity (Millipore, Brazil).
The standards of (6S)-5,6,7,8-sodium tetrahydrofolate (THF), (6S)-5-methyl-5,6,7,8-tetrahydrofolate (5-MTHF) and (6S)-5-formyl-5,6,7,8-tetrahydrofolate (5-FTHF) were kindly provided by Merck-Eprova (Switzerland) and maintained at -18oC before used.
The stock solution of the folate standards (200 μg/mL) and the solutions with increasing concentrations were prepared in an extracting solution (phosphate buffer solution 0.1 M, pH 6.0, containing 1% ascorbic acid, and 0.1% 2-mercaptoethanol).
The real concentration of the standard solution was verified by spectrophotometry and altered according to the following equation:
A = E x C x L
In which:
A = Maximum absorbance (read at 297 nm for THF, at 290 nm for 5-MTHF and at 285 nm for 5-FTHF, in phosphate buffer solution 0.1 M, pH 2.0) (8);
E = Molar absorption coefficient (for THF, 27; for 5-MTHF, 32 and for 5-FTHF, 31.5) (9);
C = Molar Concentration;
L = Width of the tray (1 cm).
The construction of the analytical curves of the folate isomers (THF, 5-MTHF and 5-FTHF) was carried out according to the concentration of the components in the vegetables. For each of the isomers six increasing concentrations of standard solutions were injected, where for THF, 5-MTHF and 5-FTHF these concentrations ranged from 0.0095 and 0.3821 μg/mL, 0.2432 and 9.7300 μg/mL, and 0.0490 and 0.9800 μg/mL, respectively.
The following vegetables were used: kale (Brassica oleracea L.), mustard (Sinapsis arvensis), spinach (Spinacia oleracea L.) and broccoli (Brassica oleracea Italica group) (florets and leaves). These vegetables were chosen due to their large consumption and availability and because they are considered important food sources of folate (7). Around 1 to 2 kg of each vegetable were collected.
The vegetables were randomly collected during the winter and spring and packed in plastic bags. The analyses of the vegetables were carried out in, at most, 48h after the collection. Four collections happened in each season evaluated, and each repetition was characterized by a weekly collection.
In the laboratory, the non-edible parts (stalks and damaged leaves) were removed, the vegetables were washed in running water and the excess was removed with paper towel and prepared as explained below.
It was employed the quartering method for the leaves of kale and mustard, and the portions diagonally disposed were grouped. One part was analyzed raw and another was analyzed after being manually sliced with a knife (slices of approximately 0.5 cm thick) (sliced kale and mustard). The kale was also analyzed on a manually torn manner. After the pre-preparation, the vegetables were stir fried. For each 100 g portion of vegetable, 8 mL of soybean oil were used (1 tablespoon). The time of cooking was 2 minutes.
The leaves of broccoli and spinach were divided in two parts, one was analyzed raw, and the other after being stir fried. For each portion of 100 g of vegetable, 8 mL of soybean oil were used and the time of cooking was 2 min.
The florets of broccoli were divided into three parts; one of them was analyzed raw, the other was analyzed after being steam-boiled, in a regular pan, for 6 min, and the third part, after being boiled, also during 6 minutes (enough quantity of water to cover the vegetables: 100 g of vegetables for each 1000 mL of water) (10).
The quantities of vegetables used for cooking were based on those used in household preparation.
Soon after being cooked, all the vegetables were blended in a food processor for a complete homogenization, and were kept in a regular refrigerator before analysis.
To evaluate stability, the folate concentration retention percentage in the vegetables was reached after using different cooking methods, taking into account the weight changes that occurred during the process. For such, it was used the formula of the true or real retention (% TR) (11), as described below:
% TR = Fcooked (μg) x Qcooked (g) x 100
Fraw (μg) x Qraw (g)
In which:
Fcooked = folate concentration in the cooked vegetable
Fraw = folate concentration in the raw vegetable
Qcooked = quantity of cooked vegetable
Qraw = quantity of raw vegetable
During the steps of extraction and analysis, the vegetables and the extracts were protected against solar and artificial light, with the use of amber glass, aluminum foil and blackout drapes, and were protected against environmental oxygen with the use of lids and with nitrogen gas in glasses.
The extraction was based on methodology previously used (12, 13, 14). For the extraction, about 3.00 g of each vegetable, previously homogenized in a food processor, were weighed in a digital semianalytical balance. The vegetable was ground with phosphate buffer solution 0.1 M, pH 6.0, containing 1% ascorbic acid and 0,1% 2-mercaptoethanol, and vacuum-filtered in büchnner filter. The volume was completed to 25 mL and the extract obtained was heated for approximately 12 minutes in water bath at 100 ºC, under agitation. Afterwards, it was cooled, centrifuged (1789 g, for 30 minutes) and then, used for the deconjugation of polyglutamates.
For the deconjugation of polyglutamates into monoglutamates, 100 μL of rat plasma containing the enzyme conjugase (γ-glutamyl carboxipeptidase) were added to the obtained supernatant (3 mL) achieved in the procedure of extraction previously described. The extracts were placed in water bath at 37°C for 3 hours. Then, for enzymatic inactivation to occur, the extracts were heated in boiling water for 5 minutes (13).
The purification of the extract was carried out based on procedure previously described (13). The extract obtained in the previous procedure was purified using a strong ion exchange column, with stationary phase of Q-Sepharose, prepared in our laboratory. The column was pre-conditioned with methanol and water (1:1) at a flux of 1-2 drops/second (using peristaltic pump). Then, the extract was applied to the column at a flux of 2 drops/second, approximately. The column was washed with ultrapure water to remove the interfering components (flux of 1-2 drops/second). After that, the elution of the folates retained was performed with sodium acetate (0.1 M) containing 10% sodium chloride, 1% ascorbic acid and 0,1% 2-mercaptoethanol.
Before the chromatographic analysis, the extract was filtered through membranes with a porosity of 0.45 μm.
The HPLC system (Shimadzu, SCL 10AD VP model) employed in the analysis of folates was composed of high pressure pump (valve for low pressure quaternary gradient pump), LC-10AD VP model; automatic injector with a loop of 50 μL, SIL-10AF model and detector of fluorescence (RF10AXL model). The system was controlled by the Multi System software program, Class VP 6.12. The separation of folates was carried out in a Shim Pack 100 RP18 column, 150 mm x 4.6 mm, 4.6 μm (Merck, Germany).
The chromatographic conditions used were: mobile phase composed of a binary gradient containing acetonitrile and phosphate buffer solution (NaH2PO4 30 mM, pH adjusted for 2.3 with H3PO4); flow 0.7 mL/min; injection volume, 50 μL, detection by fluorescence with excitation at 290 nm and emission at 360 nm. The gradient started with acetonitrile 6% (v/v), linearly increasing to 25% in 25 minutes. This concentration was maintained for 2 minutes, then, it returned to the initial conditions. The column was reequilibrated for 15 minutes before the following run. The total running time was 43 minutes.
The identification of THF, 5-MTHF and 5-FTHF in the vegetable extracts was carried out by comparing the retention times obtained in the extracts with those obtained for the respective standards analyzed under the same conditions, and by co-chromatography.
Quantification of the isomers of folates in the vegetables was performed based on the analytical curves and regression equations achieved (THF: Y = 1178396061 x + 158374.83; R² = 0.99; 5-MTHF: Y = 1634094176 x – 262055.40; R² = 0.99; 5-FTHF: Y = 119560115.8 x – 1041248.88; R² = 0.98). The real concentration in the vegetables was calculated based on the dilutions carried out.
For the study of the folate concentrations, it was considered a completely randomized design with two seasons of the year (winter and spring), five vegetables and four repetitions in each season. Analysis of variance was carried out (α= 5%) to verify the existence of significant differences between both seasons. For the evaluation of significant differences between the concentration of the compounds in raw and cooked vegetables, the paired t-test was applied, with 5% of probability. The vegetables were compared two by two (raw vegetable x cooked/stir fried vegetable). For this analysis, eight repetitions were considered (four repetitions in the winter and four in the spring).
All the statistical analyses were carried out using the SAS (Statistical Analysis System), version 9.1 (2002-2003), licensed for the Federal University of Viçosa.
The chromatographic conditions used allowed a good resolution of the folate peaks (THF, 5-MTHF and 5-FTHF), allowing for accurate quantification in the vegetables (Figure 1). All isomers were identified in the studied vegetables and the retention times were approximately 11, 14.5 and 20 min for THF, 5-MTHF and 5-FTHF, respectively.
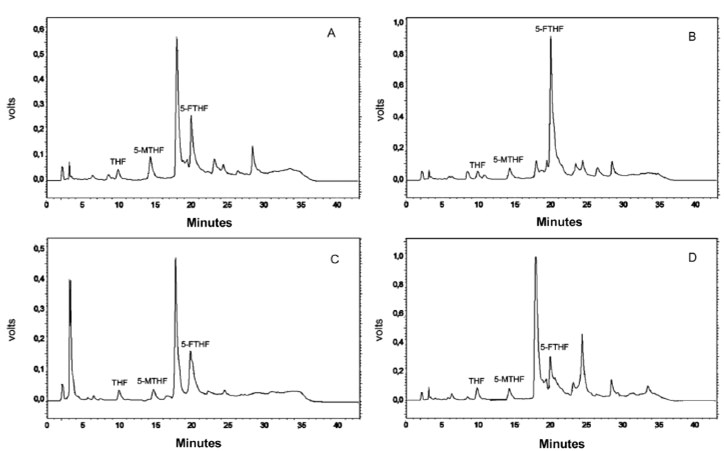
Table 1 shows the data of the folate concentration in raw vegetables.
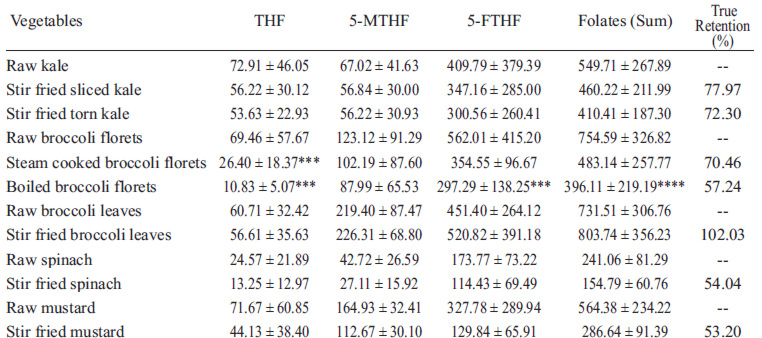
The total folate concentration varied from 154.79 μg/100 g in stir fried spinach (lowest value) to 803.74 μg/100 g in stir fried broccoli leaves (highest value).
There were no significant differences on folate concentrations between the two seasons evaluated (winter and spring) for any samples (data not shown).
The percentage of retention of each folate isoform after the use of different cooking methods for vegetables is demonstrated in Figure 2.
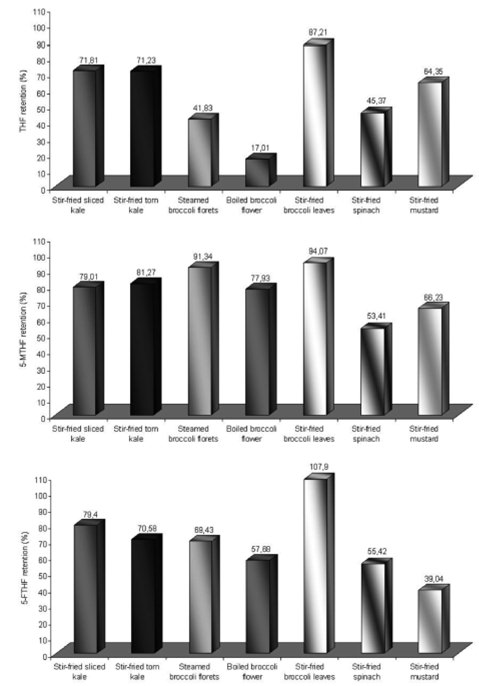
The retention of the isomers in the vegetables after being boiled varied from 17.01% to 87.21% for THF, 53.41 to 94.07% for 5-MTHF and 39.04 to 107.9% for 5-FTHF. THF was the less stable isomer, while 5-MTHF was considered the most stable for the cooking processes.
It is verified that the lowest retention occurred in florets of broccoli cooked under immersion, which preserved only around 17% of the THF concentration after they were boiled. Apparently, there was a great reduction in folate retention, even in those vegetables that did not present any statistically significant differences after cooking. The sliced kale, for example, presented a retention similar to 71.81%, 79.01% and 79.4% of THF, 5-MTHF and 5-FTHF after being stir fried, while the stir fried mustard presented a retention of 64.35% for THF, 66.23% for 5-MTHF and 39.04% for 5-FTHF
Although no studies were previously found evaluating the folate concentration in broccoli leaves, it was observed that this vegetable, commonly rejected during the pre-preparation stages, contributes with great concentrations of this vitamin. Therefore, its consumption, in soups, souffles and salads must be encouraged. Several works report vitamin concentrations in kale, but no studies were found focusing on folate concentrations in this vegetable, indicating the pioneering character of this research.
A study (14) found a total folate concentration in broccoli florets similar to that found in the present work. The authors detected a concentration of 866 μg/100g, but this value is usually higher than those verified in other works (4, 10, 12), in which the concentrations of this vitamin were 427.8 μg/100g, 114 μg/100g and 102 μg/100g, respectively.
In spinach, the total folate concentrations were in accordance with what has been previously found. A study (15) reported a folate concentration in this vegetable of 302 μg/100g. Other studies (16, 17) reported folate concentrations of 364 μg/100g and 193 μg/100g, respectively, using a similar methodology to the one used on this present study.
The only work found that deals with the folate concentration in mustard (15) detected the concentration of 278 μg/100g of this vitamin, a lower value than that found on this work.
Through the analysis of the United States Department of Agriculture table (18), it is observed that, in general, the folate concentrations found in our study were higher, except the kale, which presented lower concentrations. According to this table, the total folate concentrations for broccoli florets, kale, spinach and mustard (evaluated through the microbiological method) were, respectively, 160 μg/100 g, 764 μg/100 g, 52 μg/100 g and 137 μg/100 g.
The variation in the folate concentrations found in the present work, compared to other studies, can be explained by factors that would lead to higher or lower synthesis of this vitamin in vegetables. In fruits and vegetables, the folate isomers and their distribution in different portions are affected by light, since the synthesis of the vitamin occurs in the photorespiration (10). The degree of maturity is also important, since folates act in the cell division process and their quantity is higher in the tissues that are being divided than in the mature ones (19). Minerals such as potassium and magnesium are also very important for the fruit and vegetable production of folates, participating in one of the stages of this vitamin’s biosynthesis (12). Also, the analytical methodology in this case is very important since the results of folate content using other methods may be different from those obtained by HPLC.
It is important to emphasize that the different methodologies used for the extraction and analysis also lead to significant variations in folate concentration. The official method, based on microbiological assay, quantifies the total folate concentration present in the samples, while biospecific techniques and HPLC determine the most common isomers found, which have different roles in the metabolism.
It was observed that the boiling method presented the lowest capacity to preserve the concentration of 5-FTHF and THF in broccoli florets (see Table 1). Also, the steam-boiling was not efficient either to preserve THF in these vegetables. The stir frying method did not cause significant reduction of folate concentrations in the vegetables studied. It is important to emphasize that, for this method, the cooking time was short (2 minutes), however, the steam boiling and boiling methods occurred over a longer period of time (about 6 minutes for both methods). Besides, since folate is a hydrosoluble vitamin, it is more easily lost in boiling water. None of the methods evaluated promoted a significant reduction (p>0.05) in the concentration of 5-MTHF.
To determine if a larger surface of exposure to oxygen would promote significant losses of folates in kale, the sliced and torn preparation methods were analyzed. It is known that a larger surface area and the geometric manner in which the vegetable is cut may affect the losses of folates. However, in the present work, no effects on the retention of folates in kale caused by cutting were observed.
Our study showed that the broccoli leaves presented a concentration of folate isomers similar to those found in broccoli florets (part usually eaten). When stir fried, similarly to the other vegetables analyzed under the same conditions, the broccoli leaves did not present significant reduction in folates.
In a study on the retention of folates in foods commonly consumed by the population of the United Kingdom (1), the total folate concentrations found (evaluated by the microbiological method) in raw spinach varied from 189.5 μg/100 g to 191.8 μg/100 g, and in raw broccoli florets from 172 μg/100 g to 177 μg/100 g. After boiling, the concentration of this vitamin was significantly reduced to 94.4 μg/100 g and 102.8 μg/100 g in spinach and broccoli, respectively. Selection of the cooking methods may explain the losses since folates may be lost by lixiviation, although the folate content in the water after cooking the vegetables was not assessed in this study. On the other hand, the steam cooked broccoli florets did not present significant losses of total folates. In the present study, losses were observed in both boiling and steam boiling.
In a study regarding the effect of cooking spinach (10), a reduction in the concentration of 5-MTHF from 552.0 μg/100 g to 127.1 μg/100 g was found, different from the findings of the present study, in which the concentrations of 5-MTHF in all vegetables analyzed remained stable after boiling. Moreover, in the study developed by those authors, a reduction of 5-FTHF from 7.8 μg/100 g to 1.6 μg/100 g was observed after boiling in water. The authors did not notice the presence of THF in the vegetable studied. It has been observed that in most of the previous works, concentrations of 5-FTHF were lower than those detected in the present study.
Other authors also found considerable folate losses in spinach after boiling. In a previous study (18), authors observed losses of 83% in the concentration of total folates, while other authors (20) reported losses of 70% in the concentration of this vitamin. Although the steam boiling method was not analyzed by these authors, they suggest that this cooking method presents greater capacity to retain folates when compared to the immersed boiling method, supporting the results of the present study, where 5-FTHF retention was higher in the first cooking method.
Another study (1) verified that folate retention in spinach after boiling was 49%, and the concentrations varied from 191.8 to 94.4 μg/100g for the vegetable in natura and after boiling, respectively. Similarly, the retention of folates in broccoli was 44% (177.1 and 77μg/100g, in natura and after boiling, respectively). The steam boiling method did not cause statistically significant losses of folates for these two vegetables, and resulted in a significantly higher retention in both vegetables when compared to boiling. In our study, the steam boiling method preserved the concentration of 5-MTHF and 5-FTHF in broccoli florets, but it was not effective in the preservation of THF.
No other previous work was found regarding folate losses after cooking kale, broccoli and mustard leaves, demonstrating the importance and contribution of data gathered from the present study.
A study regarding folate retention in vegetables (21), low retention of this vitamin was indicated during cooking, possibly due to lixiviation. Sliced, frozen and fried potatoes presented half the folate concentration of raw potatoes (dry basis). Sliced carrots lost approximately 40% of their folate concentration during boiling. Although the vegetables analyzed by these authors are not similar to those in the present study, the data suggests concerns related to folate retention after the different cooking methods.
Another study (14) revealed significant folate losses in potatoes in function of the food preparation conditions. The greatest retention occurred with the sous-vide method (103%), followed by boiling (72-59%), and finally by steam cooking (63%). In the present study, the boiling (immersed in water) was not effective to preserve folates in vegetables.
The high standard deviation values observed in this study (Table 1) are common in works involving the analysis of vitamins in food matrices. Due to the great biological variability among these foods, wide data variation is expected, even when researchers attempt to reduce sample heterogeneity, collecting samples of the same variety, same cultivation location, same maturity stage, in randomly selected collection locations and complete homogenization of vegetables before the extraction procedure. In this study we attempted to minimize this bias by collecting the vegetables at the same commercialization stage, obtaining as many repetitions as possible (eight repetitions) and mixing large quantities of representative samples (1-2 kg of each vegetable) before removing aliquots for analysis. However, in the present study we worked with a real consumption situation, in which the population cannot control the acquisition of the same vegetable variety. Vegetables were obtained from different producers, who grew different varieties of the same vegetable. Thus, samples from different variety of each vegetable were obtained, which increases their variability.
In another study (10), only the isomers 5-MTHF and 5-FTHF were found in broccoli florets. Loss of these isomers after boiling in water was approximately 68%, and 53% of the concentration lost was found in the boiling water, indicating that losses were mainly caused by lixiviation. On the other hand, in other work (14) the authors observed lower folate losses in broccoli after boiling in water, approximately 24.5%. Our study reported intermediate losses of 5-FTHF in relation to both previously mentioned works, after the boiling of broccoli florets in water (retention of 57.68%).
Another study detected folate losses by lixiviation in spinach after boiling of approximately 74% for 5-MTHF and 56% for 5-FTHF (10). Approximately 58% of the 5-MTHF concentration and about 39% of 5-FTHF migrated to the boiling water. The authors reported that 20% of the total folates probably degraded during household processing (washing, slicing and heat losses). In our work boiled spinach was not analyzed, but instead stir fried, which presented lower folate isomer losses.
Differences in the retention of folates in several vegetables submitted to boiling in water were previously studied (22). The highest retention was found in Brussels sprout, cauliflower and broccoli. After 8 minutes of cooking, more than 75% of the initial concentration of 5-MTHF remained in these vegetables. Lower values of retention of this isomer were found in spinach, cabbage and carrot, varying between 37 and 52% of the initial concentration. Our work also showed the preservation of 5-MTHF, but this result was similar for all the vegetables studied, differently from the work of these authors.
Some authors consider that other cooking methods, which were not evaluated in this study, also promoted good retention (approximately 80%) of folates in vegetables, such as pressure boiling and on the microwave oven (23, 24). Moreover, the method of stir-frying is able to preserve the content of folate in vegetables compared to cooking in water due to the nature of this vitamin (water soluble).
The strong relation between the losses of folate and the duration of cooking may partly explain the losses of vitamin observed in this study and in works of other authors. In a study (25), authors observed losses of up to 78% of folates in spinach after ten minutes of boiling in water, while other (1) reported 51% of folate losses after three minutes of boiling in water. In the present work, boiled spinach was not analyzed, but broccoli florets immersed and cooked presented significant losses. It may be due to the fact that the vegetable remained for about six minutes in contact with water, in comparison to the two minutes in which the stir fried vegetables were in contact with oil.
The solubility of the folates in water is a characteristic that considerably affects their loss, mainly by lixiviation, when there is a direct contact between the vegetable and the boiling water (10). Although the vegetables in this study have presented high concentration of folates, the losses occurred during the process of boiling in water must be considered in researches about food consumption.
There were no significant differences on folate concentrations between the two seasons evaluated (winter and spring) for any samples.
Retention of folates in leafy vegetables was strongly dependent on each vegetable, the method of cooking and the isomeric form. Stir frying preserved the concentration of this vitamin in vegetables, while boiling was the method that caused the greatest losses of THF and 5-FTHF.
As the tests were conducted on laboratory scale, simulating domestic preparation, it is necessary to perform further studies to assess folate losses after the stages of handling and preparation on a large scale, as the foodservices, increasing information about the nutritional quality of vegetables consumed routinely.
The authors thank CAPES for the granting of the master’s scholarship and FAPEMIG, for the scientific initiation scholarship.
Recibido: 17-09-2013
Aceptado: 07-03-2014