The industrialization of potato and tomato produces large amount of wastes. Previous studies have demonstrated that these by-products are rich in antioxidant compounds. In this study, the composition and antioxidant efficacy of extracts from potato and tomato by-products were determined in order to evaluate their potential as food additives. Potato and tomato wastes showed to be good sources of fiber, protein and antioxidants. The antioxidant activity and total phenolic, carotenoid and lycopene contents were determined in methanol, ethanol and acetone extracts of the residues. Methanol was the best solvent for the extraction of phenolics while acetone was the best in the extraction of carotenoids in both residues. The greatest antioxidant activity (14.10 μmol Trolox/g) resulted when potato peels were extracted with ethanol. The oxidative stability of canola oil containing either ethanol extracts of potato and tomato wastes (200 and 400 mg/kg) or the synthetic antioxidant BHT (200 mg/kg), compared to oil without antioxidants, was evaluated by measuring their peroxide values, conjugated dienes and p-anisidine value after 72 and 144 h storage at 65 °C. The order of antioxidant efficacy was as follows: potato peels > BHT > tomato residues. The results showed that ethanol extracts of potato and tomato waste could be used as natural antioxidant additives in the protection of vegetable oils.
Key words: Tomato by-products, potato peels, total phenolics, total carotenoids, lycopene, antioxidant additives.
La industrialización de la papa y el jitomate genera grandes cantidades de desechos. Estudios previos han demostrado que estos subproductos son ricos en compuestos antioxidantes. En este trabajo se determinaron la composición y la eficacia antioxidante de subproductos de papa y jitomate con el fin de evaluar su potencial como aditivos alimentarios. Los desechos de papa y jitomate demostraron ser buenas fuentes de fibra, proteína y antioxidantes. Se determinó la actividad antioxidante y el contenido de compuestos fenólicos, carotenoides y licopeno en extractos metanólicos, etanólicos y acetónicos de los residuos. El mejor disolvente para la extracción de compuestos fenólicos fue el metanol mientras que la acetona fue el mejor disolvente para extraer los carotenoides. La mayor actividad antioxidante (14.10 μmol Trolox/g) se obtuvo cuando las cáscaras de papa se extrajeron con etanol. La estabilidad oxidativa de aceite de canola adicionado con los extractos etanólicos de desechos de papa o jitomate (200 y 400 mg/kg) o con el antioxidante sintético BHT (200 mg/kg), comparándolos con aceite sin antioxidantes, se evaluó mediante la medición de su índice de peróxidos, dienos conjugados e índice de anisidina, después de almacenarlo a 65°C durante 72 y 144 h. El orden de eficacia antioxidante fue como sigue: cáscara de papa > BHT > residuos de jitomate. Los resultados demostraron que los extractos etanólicos de los desperdicios de papa y jitomate podrían ser usados como aditivos antioxidantes naturales en la protección de aceites vegetales.
Palabras clave: Subproductos de jitomate, cáscaras de papa, fenólicos totales, carotenoides totales, licopeno, aditivo antioxidante.
Escuela Nacional de Ciencias Biológicas, Instituto Politécnico Nacional, México, D.F. México
Lipids can undergo a series of chemical reactions such as autoxidation, thermolysis and polymerization when they are exposed to heat, light, oxygen and other oxidizing agents (1). This results in the decrease of the nutritional value of food as well as changes in colour, texture and other sensory and physiological properties. Lipid peroxidation resulting from the reaction between the unsaturated fatty acids and molecular oxygen is a severe problem for the fat and oil industry. It not only deteriorates the quality of fat and fatty foods causing chemical damage, but also produces free radicals and reactive oxygen species that are associated with carcinogenesis, mutagenesis, inflammation, aging and cardiovascular disease. Because of this, the consumers do not accept oxidized products and, as a consequence, the food industries suffer economic losses (2).
Synthetic antioxidants, such as BHT (butylated hydroxytoluene), BHA (butylated hydroxyanisole) and TBHQ (tertiary butyl hydroquinone) have been widely used for the preservation and protection of high-fat products against oxidation. However, some studies indicate that these compounds may be involved in many health risks, including hepatic damage and cancer (3). Therefore, research has been focused on the study of naturally occurring antioxidants, such as those from agricultural by-products, as sources of food additives (4-8). The efficiency of methanolic extracts of wastes from pomegranate, apple, banana, citrus, corn, wheat, and rice for improving the oxidative stability of corn oil has been proven (5). The methanolic extracts of peels from tomato, cucumber, and watermelon also had antioxidant activity against sunflower oil oxidation (8) while extracts of potato peels and sugar beet pulp were effective in stabilizing sunflower and soybean oils (2).
Tomatoes and potatoes are two crops widely grown in the world. In 2013 the world production of tomatoes and potatoes was approximately 163 and 374 million tons, respectively (9). The industrialization of these vegetables produces large amounts of waste (20-50 kg/ton and 100-120 kg/ton of the initial weight of tomatoes and potatoes, respectively) that could be used in the production of antioxidants (10-11). Dry tomato by-products (seeds and peels) have a high content of carotenoids (approximately 950 mg/kg), mainly lycopene, lutein and β-carotene (11), as well as the phenolic compounds quercetin and kaempherol (8). On the other hand, chlorogenic, caffeic, protocatechuic, hydroxybenzoic, p-coumaric, ferulic, and gallic acids have been identified in potato peels (8,12). The high antioxidant activity of all of these compounds has been demonstrated in different studies. Phenolic compounds have an ideal structure for scavenging free radicals because they have phenolic hydroxyl groups that are prone to donate a hydrogen atom or an electrone to a free radical and have a conjugated aromatic system to delocalize the impaired electrone (1). On the other hand, carotenoids can interact with free radicals and have the ability to quench singlet oxygen due to their conjugated double bond system (8).
The objectives of this study were to determine the total phenolic compounds, carotenoids and antioxidant activity of extracts from potato and tomato wastes obtained with different solvents, and to evaluate its effectiveness in preventing the oxidation of canola oil.
ABTS (2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)|, potasium persulfate, trolox (6-hydroxy-2, 5, 7, 8-tetramethylchromane-2-carboxylic acid), gallic acid, BHT (butylated hydroxytoluene), and p-anisidine, were purchased from Sigma-Aldrich (St. Louis, MO).
Potato tubers (Solanum tuberosum L. cv. Alpha) and tomatoes (Lycopersicon esculentum Mill. cv. Saladette) were obtained from a local retail market of Mexico City. Both vegetables (20 kg each) were of a maturity state similar to that used for the industry and without any physical damage. Potatoes were washed and peeled with a mechanical peeler, whereas tomatoes were previously fractionated and heated at 90°C for 60 sec (hot break) and then sieved to obtain the by-products (peels and seeds). Both residues were then dried in a convection oven at 45°C, ground and sieved trough a 0.5 mm standard mesh. Canola refined oil without antioxidants were obtained from a local refinery of Mexico.
Moisture, crude protein, crude fat, ash, and crude fiber were determined according to the methods of AOAC (13), whereas carbohydrates were calculated by difference
Ground materials (10 g) were extracted in a shaker with 100 mL of 80% ethanol at room temperature overnight. Afterward, the extract was recovered by centrifugation at 6182 g for 15 min at 4°C, and the residue was re-extracted under the same conditions. The combined extracts were evaporated in a rotary evaporator at 40 °C. Dry extracts were weighed and stored at -20°C in the dark for further use in the oxidative stability determination. The antioxidant compounds were extracted with different solvents (80% ethanol, 80% methanol, and acetone) in order to select the best solvent to give the highest yield of antioxidants. For the determination of phenolic compounds, carotenoids, lycopene, and antioxidant activity, extracts were used without evaporation.
Total phenolic content was determined using the modified Folin-Ciocalteu method (14). A 0.5 mL aliquot of extract solution was mixed with 4.5 mL of distilled water and 0.5 ml of Folin-Ciocalteu reagent, and allowed to react at room temperature for 3 min. Then, 1 mL of 1N sodium carbonate was added, and the mixture was incubated at room temperature for 1 h. The absorbance was measured at 725 nm. Gallic acid was used as standard and total phenolic content was expressed as milligrams of gallic acid equivalents (GAE) per 100 g dry weight (DW) sample.
Carotenoids and lycopene contents were determined according to the methods of Scott (15), and Fish et al. (16), respectively, with slight modifications. In brief, 10 mL of sample extract was added to 10 mL of hexane into an assay tube previously wrapped in aluminum foil. The tube was introduced in an ice bath and stirred in an orbital shaker at 180 rpm for 15 min. Then, 3 mL of deionized water was added to each tube, and the samples were shaken for 5 min more. Shaking was stopped, and tubes were left at room temperature until phase separation. The absorbance of the hexane layer (A) was measured at 450 nm for total carotenoids determination, and at 503 nm for lycopene content, using a blank of hexane. The total carotenoid (TC) concentration was calculated as follows: TC (mg/kg) = (A x V x 104) / (A1% x m); where A is the absorbance at 450 nm, V is the hexane volume, A1% is the extinction coefficient for total carotenoids (2500), and m is the weight of the sample in the extract. The lycopene content was estimated by the relation: Lycopene (mg/kg) = A x 31.2 / m; where A is the absorbance at 503 nm, and m is the weight of the sample in 10 mL of solvent.
The ABTS radical-scavenging activity was determined according to the method of Re et al. (17). The ABTS•+ stock solution was prepared by mixing 7 mM of ABTS with 2.45 mM of potassium persulfate (final concentration), and allowed to react at room temperature in the dark for 12-16 h. The stock solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm. Extracts, synthetic antioxidant (BHT) or Trolox standard solutions (20 μL) were allowed to react with 1980 μL of ABTS•+ for 7 min, and then the absorbance was measured at 734 nm. Ethanol (80%) was used as a blank. The antioxidant activity was expressed as μmol of Trolox equivalents (TE) per gram of dry weight.
The Schaal method was used to evaluate the effect of the extracts against canola oil oxidation (2,18). The extracts of potato peels and tomato residues were applied to refined canola oil (free of antioxidants) at different concentrations (200 and 400 mg/kg of dry extract). BHT at a level of 200 mg/kg was also applied for comparison. An oil sample, without antioxidant, was used as a control. Glass jars (50 mL, wide mouth, with screw caps) were filled with 30 mL of the test samples and were capped. The jars were subjected to accelerated oxidative storage in an oven at 65°C for 144 h. Peroxide value, conjugated dienes, and anisidine value were evaluated in triplicate at zero time, 72 and 144 h.
The peroxide value (PV) was determined iodometrically following the AOAC official method (13).
To measure the conjugated dienes, the specific extinction at 232 nm was determined using a spectrophotometer. Previously, the oil samples were diluted with iso-octane to bring the absorbance within limits (0.2-0.8).
The p-anisidine value (AV) was determined according to Cd 18-90 method of AOCS (19). Briefly, 0.2 g of oil samples were dissolved in 25 mL iso-octane and absorbance of this solution was measured at 350 nm using a spectrophotometer. Five milliliters of the above mixture was mixed with 1 mL of 0.25% p-anisidine in acetic acid (w/v) for 10 min, and absorbance was read at 350 nm. Anisidine value was calculated according to the equation: AV = 25 x (1.2As – Ab)/ m; where As is the absorbance of the fat solution after reaction with the p-anisidine reagent, Ab is the absorbance of the fat solution, and m is the mass of the oil sample (g).
All analyses were performed in triplicate. The results were expressed as the mean ± standard deviation, and significance was determined by ANOVA and Tukey post-hoc test using Microsoft Excel 2007 and the methods described by Montgomery (20). Differences were considered statistically significant if p ˂ 0.05. Pearson’s correlation coefficient was also calculated using Excel.
Table 1 shows the proximate composition of tomato and potato by-products. Both wastes had high protein and fiber contents. However, tomato waste had higher fiber and fat amounts and lower ash and carbohydrate contents than potato peels (p˂0.05), whereas there was no difference in the protein content of both residues (p>0.05).
Table 2 shows the contents of total phenolic compounds, carotenoids and lycopene as well as the antioxidant activity of the samples. The amount of phenolic compounds varied in the different extracts, ranging from 11.46 to 79.20 mg GAE/100 g DW for potato peels, and from 3.23 to 43.43 mg GAE/100 g DW for tomato residues depending on the solvent used to extract them. The results indicated that ethanol and methanol were better than the acetone (p˂0.05) to extract phenolic compounds from these kind of materials.
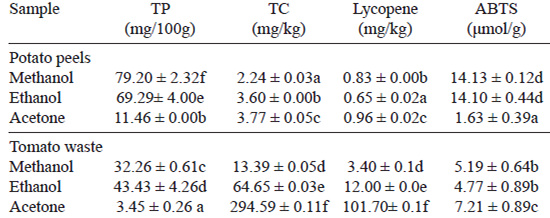
In the case of total carotenoid and lycopene content, acetone was better solvent than methanol and ethanol to extract these components. Total carotenoids were in the range of 2.24 to 3.77 mg/kg for potato peels, and 13.39 to 294.59 mg/kg for tomato wastes, whereas lycopene varied from 0.65 to 0.96 mg/kg for potato peels, and from 3.4 to 101.7 mg/kg for tomato wastes, depending on the solvent used in the extraction.
The extracts obtained from potato peels showed to be more efficient ABTS radical scavengers (14.13,14.10, and 1.63 μmol trolox/g) than extracts obtained from tomato waste (5.19, 4.77, and 7.21 μmol trolox/g), using methanol, ethanol and acetone. Methanol and ethanol were better solvents to extract antioxidants from potato peels than acetone while acetone was better for tomato by-products. It is worth noting that there was no significant difference (p>0.05) between the ABTS activity of the methanol extracts and the activity of ethanol extracts in both wastes.
There was a strong positive correlation between antioxidant activity (ABTS) of potato peel extracts and its total phenolic content (r=0.99; p˂0.0001) but no significant correlation was observed with respect to total carotenoids, and it was negative with respect to lycopene content (r=-0.81 (p˂0.05).
On the contrary, the correlation coefficient between antioxidant activity and total phenolics in the tomato waste extracts was negative (-0.90; (p˂0.001) while was positive with respect to total carotenoid and lycopene contents (0.83 (p˂0.01), and 0.74 (p˂0.05), respectively).
The antioxidant efficacy of the ethanol extracts towards the stabilization of canola oil was examined. The extract yields obtained with 80% ethanol were 11.97% of potato skins extracts and 9.9% of tomato waste extracts. Peroxide value, conjugated dienes and anisidine value were determined to evaluate the extent of lipid oxidation during the accelerated storage of canola oil at 65°C.
An increase in PV with the increase in the storage time was observed in all samples (Figure 1A). The rate of peroxides formation was slower in the first 72 h with respect to the following 72 h, showing an induction period. A statistically significant diminution of the PV of 21.1, 67.7, 20.0, and 26.3% in canola oil samples added with 200 and 400 mg/kg of potato peel extracts (P200 and P400), and 200 and 400 mg/kg of tomato waste extracts (T200 and T400), respectively, was observed after 72 h of storage time. The PV of canola oil added with BHT was increased by 9.9% at this storage time.
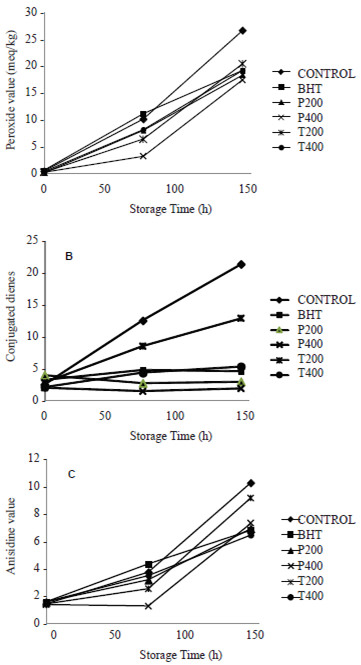
Canola oil samples containing either potato peel extracts (200, 400 mg/kg) or tomato waste extracts (200, 400 mg/kg) and BHT (200 mg/kg) reached maximum peroxide values of 18.42, 17.45, 20.45, 19.23, 19.24 meq/kg, respectively. These values were significantly lower than those obtained in the canola oil without antioxidants which had a maximum PV of 26.69 meq/kg after 144 h of accelerated storage. The PV of oils added with P200 and P400 were significantly lower and that of the oil added with T200 higher than the PV of the oil with BHT (p˂0.05) while there was no difference between the PV of the oil added with T400 and that of the oil with BHT (p>0.05).
Figure 1B presents the relative increase in conjugated dienes (CD) content in the oil samples as function of storage time at 65°C. The highest content of conjugated dienes was observed in the canola oil free of antioxidants which reached a maximum value of 21.47 after 144 h of storage. This was followed by T200 (12.96), T400 (5.35), BHT (4.56), P200 (2.98) and P400 (1.89). The differences with respect to control without antioxidants were highly significant (p˂0.0001). However, the CD content of oils added with tomato waste extracts was lower than that of the oil with BHT (p˂0.01) whereas the CD content of oils added with potato peel extracts was higher than that of the oil with the synthetic antioxidant (p˂0.001).
Figure 1C shows the p-anisidine value for canola oil samples stabilized with ethanolic extracts of potato and tomato by-products, BHT, and control. Control oil reached a maximum value of 10.27 after 144 h of storage while BHT, P200, P400, T200 and T400 reached average values of 6.82, 6.94, 7.34, 9.16, and 6.46, respectively. The differences with respect to control without antioxidants were highly significant (p˂0.01). There was no difference between BHT and P200 (p=0.41) while oils added with P400 and T200 had AV higher than the oils added with BHT (p˂0.05), and the oils added with T400 had AV lower than the oils with the synthetic antioxidant (p˂0.001).
There are already some published works about the potential of potato and tomato waste as antioxidants (2,5,6,8). However, this study provides new data that can help to make sustainable the industrialization of these vegetables. In this work, information about the antioxidant potential of by-products from potato and tomato of botanical varieties not reported by other studies was obtained. Moreover, the relative differences between the two by-products in terms of the nature of the compounds responsible for their antioxidant activity were established.
The proximate analysis of potato and tomato byproducts showed that these residues are good sources of fiber and protein. A food product is considered a “good source” of any nutrient if it provides 10-19% of the recommended dietary allowance (21). The consumption of 35 g of these by-products provides more than 10% of the dietary reference intakes of protein and fiber in a diet of 2500 kcal. The potential health benefits of dietary fiber are well documented. The consumption of dietary fiber reduces the risk of developing diseases such as diabetes, obesity, coronary and cardiovascular diseases, gastrointestinal disorders, hypertension, strocke, and colon cancer (22). The composition of potato peels determined in this work differed from that obtained by Mohdaly et al. (2) who found values of 6.55% moisture, 8.46% crude fat, 13.9% crude protein, 8.48% ashes, 13.0% crude fiber, and 56.2% of carbohydrates, in potato peels from Diamond variety. These differences could be attributed to variations in culture conditions, method of peeling, and potato variety. On the other hand, the composition of tomato residues was similar to that found by Knoblich et al. (11) for tomato seeds (20.2, 6.4, 53.8, and 5.2% for protein, fat, fiber and ashes, respectively).
Ethanol and methanol were better than acetone to extract phenolic compounds from the potato and tomato residues. It is known that the extraction efficiency of polyphenols depends on the solvent polarity and on the molecular weight of phenolic components among other factors (1). The low molecular weight polyphenols, which predominate in the potato and tomato residues, are best extracted with methanol and ethanol which have higher polarity than acetone. Hanson et al. (23) found values ranging between 29 and 161 mg GAE/100 g in different varieties of tomatoes while Nawal et al. (8) and Samarin et al. (6) obtained 39 and 52.2 mg GAE/100 g potato peels, respectively, using methanol as solvent.
On the other hand, acetone was better solvent than methanol and ethanol to extract the carotenoid components of the samples, probably due to their lipidic nature. Lipids are more soluble in less polar solvents. The higher amount of these compounds was found in tomato residues. Tomato and their by-products are considered an important source of carotenoids and the major source of lycopene (8,11). In the present work, a maximum of 294.59 mg/kg total carotenoids and 101.72 mg/kg lycopene was obtained using acetone as solvent. Knoblich et al. (11) reported 734 mg/kg lycopene in tomato peels and 130 mg/kg in tomato seeds.
Antioxidant activity of the extracts was determined by ABTS method which was chosen because it can be applied to determine antioxidant capacity of both hydrophilic and hydrophobic antioxidants of plant extracts (1). The extracts obtained from potato peels showed to be more efficient ABTS radical scavengers than extracts obtained from tomato waste although the latter had the highest levels of carotenoid compounds. This was due to that phenolic compounds apparently contributed more to ABTS scavenging activity than carotenoid compounds. The highest antioxidant activity was obtained from the extracts with 80% methanol and with 80% ethanol, while the values obtained with acetone (in which predominate carotenoid compounds) were very low. The Pearson’s correlation coefficients indicate that polyphenols are largely responsible for the antioxidant activity of potato peel extracts while carotenoids and lycopene are responsible for the antioxidant activity in tomato residues. The main antioxidant compounds that have been identified in potato peels are caffeic acid, chlorogenic acid, protocatechuic acid, para-hydroxybenzoic acid, and gallic acid, while in tomato peels are trans-lyco-pene, cis lycopene, β-carotene, lutein, quercetin, and kaemperol (8), probably all of them contributed to the antioxidant activity of the extracts. Due to the the high content of fiber and antioxidants of potato and tomato residues they could be used as ingredients of functional foods.
Because of their major efficiency, economy and low toxicity, the ethanol extracts were chosen to evaluate the antioxidant effectiveness of potato peels and tomato by-products during oxidation of canola oil. The extract yields obtained with 80% ethanol were 11.97% of potato skins extracts and 9.9% of tomato waste extracts. These yields were higher than those obtained by Mohdaly et al. (24), and Samarin et al. (6) who obtained 10.15% and 5.65%, respectively, of ethanol extracts from potato peels, and Nawal et al. (8) who found 8.14% of methanol extracts from tomato peels.
To evaluate the antioxidant efficacy of the extracts in the stabilization of canola oil, the peroxide value, conjugated dienes and anisidine value were determined under accelerated storage. Peroxide value (PV) is one of the most used tests for the measurement of the peroxides and hydroperoxides concentration formed in the primary steps of lipid oxidation (24). The results showed that the most efficient extract was that obtained from potato peel in a concentration of 400 mg/kg, being superior to the BHT synthetic antioxidant. It has been shown that the flavor scores of oils aged four days at 60°C are equivalent to scores of oils aged four months at room temperature (18). Sunflower oil develops off-flavors at a PV of 13 meq/kg. In the present work, the canola oil free of antioxidants reached this value in approximately 3.5 days of treatment (equivalent to 3.5 months of storage at room temperature), while the oil with 400 mg/kg of potato peel extract (that had the best performance) reached this PV in 5.5 days (5.5 months at room temperature).
Although PV is a widely used method to determine the lipid autoxidation, these results were confirmed with other oxidation indices such as conjugated dienes and anisidine value.
The hydroperoxide formation in the chain of a polyunsaturated fatty acid starts with the hydrogen abstraction or the addition of an oxygen radical. This causes the displacement of a double bond towards the carbon of the adjacent methylene group, resulting in the formation of a conjugated diene (25). The measurement of the UV absorbance at 232 nm (corresponding to conjugated dienes) is thus a good indicator for evaluating the initial oxidation process of a fat. The greater the levels of conjugated dienes, the lower the oxidative stability of the oil. The results demonstrated a remarkable diminution in the generation of conjugated dienes in canola oil added with the extracts of potato and tomato by-products. Again, the extracts from potato peels had better performance than the synthetic antioxidant BHT.
PV and CD only indicate the extent of oxidation in the initial stages of lipid oxidation. Hydroperoxides, the primary oxidation products, are readily decomposed to form a mixture of volatile compounds, mainly aldehydes. In this work the p-anisidine (AV) method was used to determine the secondary oxidative products of lipid oxidation, particularly 2-alkenals and 2,4-dienals. It has been demonstrated that AV is highly correlated with overall odour intensity in heated oils (26). AV, like PV and CD of all oil samples added with the antioxidant extracts, was lower than the control sample, thus showing that the extracts of potato and tomato waste are efficient in protecting the oils against oxidation.
From the results of the present study, it is clear that ethanol extracts of potato and tomato waste can be a good alternative to replace the synthetic antioxidants used in the protection of vegetable oils and fatty foods against rancidity. The phenolic compounds rather than carotenoids appear to be responsible for the antioxidant activity of these vegetable wastes. On the other hand, the dry by-products from the industrialization of potatoes and tomatoes are good sources of fiber, protein and antioxidants which could be used as functional food ingredients.
This work was financed by Instituto Politécnico Nacional, México (Project SIP20141059).
Recibido: 11-10-2015
Aceptado: 31-12-2015