This study investigated the antioxidant activity, inhibitory effects on α-glucosidase, angiotensinconverting enzyme (ACE) and aldose reductase (AR) of five types of corn cultivars (white, yellow, black, red and purple). The total phenolic content (TPC) and total anthocyanins (TA) ranged from 146.28 to 598.71 mg GAE/g and from 1.66 to 446.38 mg C3G/100 g respectively. All tested extracts were capable of scavenging peroxynitrite (ONOO-) at level of 1.6 mg/mL of TPC to extents ranging from 41.29 to 86.23%. All of the extracts also inhibited the activity of α-glucosidase with efficacies values from 17.75 to 69.83% whereas moderate inhibitory activity ACE (17.22-42.4%) and AR inhibitory activity from 22.7 to 87.2% was shown. Differences in inhibition of peroxynitrite formation and inhibition of enzymatic activities appeared to be dependent on the profile of total phenolic compounds, anthocyanins and another non phenolic compounds present in each type of corn
Key words: Bioactivity, peroxynitrite inhibition, total phenolic compounds, corn.
La actividad antioxidante, efectos inhibitorios sobre las enzimas α-glucosidasa, enzima convertidora de angiotensina (ECA) y aldosa reductasa (AR) de cinco tipos de maíz (blanco, amarillo, negro, rojo y morado) fueron analizadas. El contenido de compuestos fenólicos totales (CFT) y antocianinas totales (TA) se encontraron de 146,28 a 598,71 mg GAE/g y de 1,66 a 446,38 mg C3G/100 g respectivamente. Todos los extractos probados fueron capaces de inhibir la formación de peroxinitrito (ONOO-) de 41,29 a 86,23% a una concentración de CFT de 1.6 mg/mL. Todos los extractos también fueron capaces de inhibir la actividad de α-glucosidasa con eficacias de inhibición de 17,75 a 69.83% y una actividad inhibidora de AR de 22,7 a 87,2%, mientras la actividad de inhibición sobre ACE se mostró moderada (17,22 a 42,4%). Las diferencias de la inhibición de la formación de peroxinitrito y de las actividades enzimáticas parecen ser dependientes del perfil de compuestos fenólicos totales y antocianinas además de otros compuestos de naturaleza no fenólica presentes en cada tipo de maíz.
Palabras clave: Bioactividad, inhibición de peroxinitrito, compuestos fenólicos totales, maíz.
1 Universidad Autónoma del Estado de México, Toluca, México. 2 Centro de Investigación en Alimentación y Desarrollo, Chihuahua, México. 3 CONACYTCentro de Investigación en Alimentación y Desarrollo, Culiacán, Sinaloa, México.
Peroxynitrite (ONOO-) possesses strong oxidizing properties toward various cellular constituents such as lipids, amino acids and DNA, besides the inhibition of the enzymes involved in maintaining genetic integrity and cell damage; their overproduction are implicated for pathological conditions such as stroke, atherosclerosis, cancer and diabetes (1). Evidence has suggested that diabetic patients are under oxidative stress, which may partially mediate the initiation and progression of diabetes-associated complications (which is one of the major metabolic disorders in people diagnosed with prediabetes or diabetes) due to the absorption of glucose released in the small intestine by α-glucosidase, such increase could be controlled by the inhibition of the enzyme. For instance, the consumption of natural inhibitors from the dietary constituents could be an effective therapy for managing hyperglycemia postprandial with minimal side effects (2). Furthermore one of the main macrovascular complications of diabetes is hypertension. One of the key actions of ACE is the regulation of blood pressure together with the water and salt metabolism, since it cleaves angiotensin I into the potent vasopressor angiotensin II. The result of ACE action is an elevation of blood pressure (3). On other hand, in hyperglycemia condition, the excess of glucose will not only be metabolized by glycolysis, but the polyol pathway in which glucose is converted to sorbitol by the catalytic activity of AR. Previous studies have provided evidence related to the involvement of AR in diabetic complications namely neuropathy, retinopathy, nephropathy and cataract (4). Modulation of ACE and AR by phenolicbased dietary ingredients may be a strategy to manage the possible diabetic complications such as hypertension and cataracts.
Corn (Zea mays L.) is one of the most important grains that provide food for most of world population. It is a source of macro and micronutrients and also is rich in phytochemicals such as phenolic acids and anthocyanins among others compounds (5). The antioxidant properties of phenolic compounds from corn have been associated with bioactivity for hyperglycemia and hypertension management (6), therefore extracts of corn might have the potential to control some effects of postprandial hyperglycemia. For the above mentioned, the aim of the present work was to characterize five corn cultivars grown in Mexico in relation to the total phenolic compounds and anthocyanins contents, and to evaluate their contribution to the antioxidant activity, and the inhibition of α-glucosidase, ACE and AR using in vitro methods.
The types of corn used in this study were yellow, white, red, purple and black. Types white and yellow were obtained from a local market in Veracruz and Oaxaca, respectively. The purple and red types were purchased from a local market in Toluca City (Estado de México), whereas the black type was obtained in local market in Mexico City, during the years 2014 and 2015. Kernels were sun-dried to a water content of ca. 20%; all grains samples were milled into whole grain flour using a 60-mesh size screen, thoroughly mixed and stored at 4oC.
Five grams of flour was extracted with 25 mL of 95% ethanol in amber glass bottles at 4oC for 24 h with constant stirring. The resulting slurry was filtered through a Whatman No. 4 filter paper and subjected to rotary evaporation (Buchi rotavapor R 110, Flawil, Switzerland) at 40oC to remove the solvent followed by lyophilization during 72 h at 13.3 Pa. Lyophilized extracts were stored in amber glass vials at 4oC.
TPC of the extracts was determined with modifications according to (7). Briefly, 15 µL of the extract were mixed into a 96-well Costar® flat bottom microplate with 240 µL of distilled water and 15 µL of 2N Folin-Ciocalteu reagent, after incubation for 3 min, 30 µL of 4N Na2CO3 were added to neutralize the reaction mixture, and then the resulting mixture was allowed to stand in the dark for 2 h. The absorbance was measured at 725 nm using a Synergy HT Absorbance Microplate Reader (BioTek Co., USA). A standard calibration curve was prepared using gallic acid and TPC in each extract was calculated and expressed as milligram of gallic acid equivalent per 100 g of dry sample (mg GAE/100 g).
Analysis of total anthocyanins was carried out according to the method of Abdel-Aal and Hucl (8), by measuring the absorbance of ethanolic extracts at pH 1.0. One gram of ground whole grain sample was homogenized in a 50 mL centrifuge tube with 25 mL of an acid–ethanol solution (0.225 M HCl in ethanol–water (95:5, v/v)). The tube was flushed with nitrogen gas, agitated for 30 min and then centrifuged at 3000 x g (Sorvall RC5C, Sorvall Instruments, Dupont, Wilmington, DE) for 15 min and the supernatants collected. Absorbance readings at 535 nm were taken and corrected for background absorbance at 700 nm (due to turbidity) in a photodiode array spectrophotometer (Model 8452A; Hewlett–Packard Co., Waldbronn, Germany)). Anthocyanins were expressed as mg of cyanidin 3-glucoside equivalents per 100 g (mg C3G/100 g) using a molar extinction coefficient of 25,965 cm-1 m-1 and a molecular weight of 449.2 g/mol.
Peroxynitrite was synthesized according to Rehman et al. (9). Briefly, 20 mL of an acidic solution (0.6 M HCl) of H2 O2 (0.7 M) was mixed with 20 mL of KNO2 (0.6 M) on ice for 10 s, and the reaction was quenched with 20 mL of icecold NaOH (1.2 M). Residual H2 O2 was removed by adding 10–15 mg of MnO2. The solution was then filtered and frozen overnight at -20oC. The yellow top layer formed by freeze fractionation was separated by scraping, and the concentration stock of ONOO- was determined at 302 nm, using a molar extinction coefficient of 1670 cm-1 m-1. Peroxynitrite scavenging was followed by inhibition of nitration of tyrosine by peroxynitrite. For each extract, a dilution series (0.2–2.0 mg/mL) and a control solution without sample were prepared. Tyrosine (10 mM) was prepared by dissolving 18.1 mg of the pure compound in 8 mL of water adding to 250 µL of 10% KOH, followed by neutralization with 250 µL of a 5% phosphoric acid solution with 1.5 mL of water. Tyrosine solution (100 µL), together with 10 µL of sample (0.1–1.0 mg/mL), were added to a plastic test tube containing 880 µL of buffer (500 mM KH2 PO4 , pH 7.4) and pre-incubated in water bath at 37o C, for 15 min. After this time, peroxynitrite (10 µL) was added under vigorous stirring (20 s), and the resulting mixture (pH 7.4–7.5) was incubated at 37oC for another 15 min. DL-Penicillamine was used as positive control. Measurement of 3-nitrotyrosine was performed using a LiChrospher RP-18 (150 mm x 4 mm, 5 μm) column. The mobile phase was 500 mM KH2PO4-H3PO4, pH 3.0 with 20% methanol (v⁄v) at a flow rate of 1 mL/min. Detection was achieved with a UV detector set at 274 nm. Peak heights of 3-nitrotyrosine were measured and their concentrations calculated from a standard curve.
The inhibitory properties of the corn extracts against α-glucosidase were assayed according to Matsui et al. (10), with some modifications. The appropriate dilution of crude extract (1.0-7.0 mg/mL TPC) and 100 µL of α-glucosidase solution (1.0 U/mL) in a 0.1M phosphate buffer (pH 6.9) was incubated at 25oC for 10 min. Then, 50 µL of 5mM ρ-nitrophenyl –α-D-glucopyranoside solution was added to the 0.1M phosphate buffer (pH 6.9). The mixtures were incubated at 25oC for 5 min prior to reading the absorbance at 405 nm in a photodiode array spectrophotometer. Acarbose (0.44 mg/mL in final assay mixture) was used as a reference inhibitor. Controls were representative of the 100% of enzymatic activity, were conducted in an identical fashion replacing the sample with buffer.
ACE inhibitory activity of the extracts was determined using the method of Hayakari et al. (11) with some modifications. Briefly, 50 µL (1.5-13.0 mg/mL total phenolic content) were dissolved in 200 µL of 0.1 M borate buffer containing 0.3M NaCl (pH 8.3) and mixed with 50 µL ACE solution (2 mU/mL). The mixture was then preincubated at 37oC for 10 min, after this time 150 µL of 5.0 mM substrate (hippuryl-histidyl-leucine) was added. The enzyme-substrate mixture was incubated for 60 min at 37oC and then 500 µL of 0.5 N HCl was added to stop the reaction. The hippuric acid produced was extracted by adding 1.5 mL ethyl acetate and stirring vigorously during 1 min. After separation by standing during 5 min, 800 µL of the ethyl acetate layer was transferred into a 2 mL Eppendorf tube and dried in a hot water bath with nitrogen flow. After drying, 1 mL of distilled water was added and the absorbance was determined in a photodiode array spectrophotometer at 288 nm. Blank samples were prepared without addition of enzyme, and control samples were prepared without the addition of extracts. Captopril (1.0 mg/mL in final assay mixture) was used as a reference inhibitor. ACE inhibitory activity was calculated using the following equation:
The aldose reductase inhibitory effect was evaluated according to Karasu et al. (12) with some modifications. Porcine lenses (20 g), supplied by a slaughter house located in Mexicaltzingo, Mexico; were homogenized with 100 mL of 0.067 M phosphate buffer (pH 6.2 at 4°C). The homogenate was centrifuged at 10 000 x g during 15 min and supernatant (crude aldose reductase extract) was collected and stored at 4°C until use. Aldose reductase was assayed spectrophotometrically by determining NADPH consumption at 340 nm and was expressed as decrease of the optical density. The reaction mixture contained 4.67 mM D,L-glyceraldehyde as a substrate, 0.11 mM NADPH, 0.067 M phosphate buffer, pH 6.2 and 100 µL of the crude enzyme extract in a total volume of 3 mL. The reference blank contained all the above reagents except the substrate D,L-glyceraldehyde to correct for the oxidation of NADPH not associated with reduction of the substrate. The reaction was initiated by adding the D,L-glyceraldehyde, and the resulting mixture was incubated at 37°C for 20 min. The reaction was stopped by adding 5 M ammonium chloride (20 µL) and cooling the mixture. The inhibitory effect on aldose reductase was evaluated by adding 100 µL of corn extracts to the reaction mixture. The aldose reductase inhibitory activity was expressed as the percentage of inhibition using quercetin as a reference inhibitor (1.0 mg/mL).
Data were reports as mean + standard deviation (SD) for three replicates.
All experiments used completely randomized block designs and significant differences between treatment means was established using one-way ANOVA and a significance level of 0.05 with the statistical software MINITAB® v. 14.
TPC and TA of the isolates from raw yellow, red, purple and black corn are shown in Table 1. The range was from 215.05 to 588.4 mg GAE/100 g and differed significantly among varieties (p>0.05), the extracts of purple type displayed higher content followed by red, black, yellow and white varieties. The TA of raw corn varied from 1.25 to 446.3 mgC3G/100 g. The purple type contained the highest content of TA, whereas the white, yellow, red and black counterparts contained approximately 0.28, 0.37, 32.6 and 54.1%, respectively of the amount of anthocyanins found in purple corn.
Table 1 shows the pattern of inhibition of peroxynitrite, mediated by inhibition of nitrotyrosine formation of the corn extracts. Crude extracts from yellow corn showed the highest level of inhibition (86.2%) followed by white corn (74.8%). The activity of the pigmented varieties ranged from 41.91 to 66.3%. The extract from red corn had the lowest scavenging potential. This ability in maize kernel extracts was not correlated to the content or total phenolic compounds (r2 =0.52).
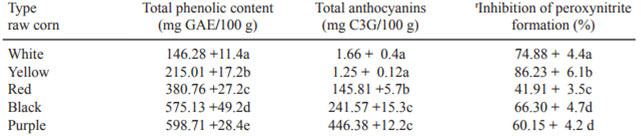
The different corn types were evaluated in relation to the possible inhibition of α-glucosidase enzyme (Figure 1). The inhibition shows the dosedependent trend to the different corn extracts. Significant inhibition against α-glucosidase was exhibited by all extracts, α-glucosidase activities ranging from 17.75 to 69.83% at the highest sample dose (7 mg/mL), the extract from yellow corn showed the highest α-glucosidase inhibition (69.8%) followed by white, black, purple and red extracts (60.62, 20.56, 18.20 and 17.48%) respectively. The inhibitory effect of the most potent corn extracts competed the effect of the acarbose (74 %)
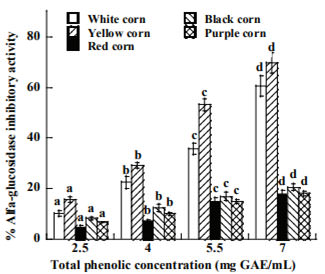
The inhibitory activity of ACE is depicted in Figure 2, the activity increased proportionally to extract concentration. Pigmented corn extracts showed the lowest inhibitory activity, which ranged from 17.23 to 20.1%, while non pigmented corn were two fold as high than pigmented corn (35.7 and 42.4 %) for white and yellow varieties. However, those activities are lower than the synthetic drug utilized in control of hypertension captopril (90.3%).
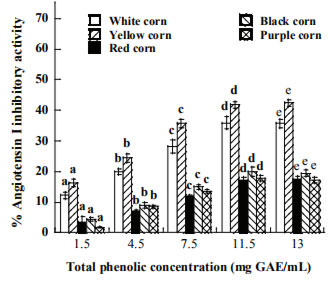
The profile of AR inhibition is presented in Figure 3. The inhibitory effect of the extracts increased with the increase of total phenolic concentration. Among the extracts, purple corn (87.20%) exhibited highest AR inhibitory activity followed by black, red, yellow and white that showed the lowest inhibition (22.72%) at the maximum dose (15 mg/mL). Extracts from purple corn at 15 mg/mL could compete with quercetin a standard inhibitor of aldose reductase that showed 96.3% of inhibition.
TPC, TA and inhibitory activity of extracts varied considerably depending on type of corn. The content of TPC differs to those previously reported. Lopez-Martinez et al. (13), found a total phenolic content in the range of 290.20 to 465.9 mgGAE/100 g of five varieties of corn, whereas Moreno et al. (14) reported lower values in different cultivars of corn ranged from 21.3 to 90.4 mg GAE/100 g. Anthocyanins are the main compounds responsible for the color in pigmented corn and are located in pericarp or aleurone, or pericarp and aleurone.
The non-pigmented types of corn (yellow and white) had the lowest anthocyanins levels; this can be explained by their colorations, which indicate the presence of carotenoids such as lutein and zeaxanthin, the most abundant pigments in this type of corn (15). Thus, from types of corn examined those that contained an elevated content of total phenolic compounds showed an elevated content of total anthocyanins. The observed differences among studies in total phenolic content and anthocyanins could be attributed to various factors such as genotype, agronomic practices, maturity at harvest, and storage condition (16).
In our previous studies we concluded that corn possess antioxidant activity (5, 13). Therefore, a better understanding of antioxidant capacity in corn would be possible by considering the inhibition of peroxynitrite formation induced by inhibition of tyrosine nitration. Crude extracts for non pigmented corn were higher in inhibition of peroxynitrite formation compared to those extracts from pigmented ones. The extract from yellow corn was the type with the highest scavenging potential among all varieties tested. Soluble compounds in water such as some flavonoids, ferulic acid and ρ-coumaric acid, react with peroxynitrite either by directing the nitration to their own structures or by deactivation by electron donation (17). Besides the presence of non phenolic components in non pigmented corns such as carotenoids and tocopherols which are ethanol extractable can scavenge peroxynitrite radicals (18), this could explain that white and yellow corn possess higher inhibition activity than pigmented varieties.
α-glucosidase inhibitors are currently used to reduce glucose postprandial plasma level in type 2 diabetes and in case of obesity. Similar results were reported by (6), they evaluated 32 varieties of corn and found that yellow samples overall had the highest α-glucosidase inhibition (72.5%), Lee et al. (18) also observed a similar behavior in 18 corn strains where yellow samples showed the highest α-glucosidase inhibition (50%) and the pigmented samples had inhibitory capacity ca. 25%. The nonpigmented varieties contained ferulic acid as the major phenolic compounds, which have been reported as strong α-glucosidase inhibitor (19).
Similar to our study Ademiluyi et al. (20) reported that the ACE inhibitory activity in phenolic –rich extracts of soybean were not associated with their phenolic content. Hellströn et al. (21) reported weak ACE inhibitory activity for chokeberry juice. Besides phenolic compounds, the ACE inhibition showed for the corn extracts could be due to the presence of other soluble components including peptides, which possess ACE inhibitory activities.
The current study aimed to identify potential AR inhibitors from corn extract that would be useful in the treatment of diabetic complications. Differences in inhibition of AR among the pigmented and non-pigmented varieties could be related with the phenolic compounds on each extracts. Yawadio et al. (22) isolated from black pigmented rice cyanidin 3-glucoside, peonidin 3-glucoside and ferulic acid, they found that the inhibitory activity of isolated compounds was as follows: cyanidin 3-glucoside>ferulic acid>peonidin-3-glucoside. Non pigmented genotypes of corn possesses high concentration of free ferulic acid (23) while the pigmented varieties have high concentration of anthocyanins (5), this could explain the different inhibitory effect between pigmented and no pigmented varieties.
The crude extracts obtained from the different types of corn have several potential beneficial effects, including, inhibition of α-glucosidase, ACE and AR. There are considerable differences in the content of total phenolic compounds, total anthocyanins, inhibition of peroxynitrite formation, α-glucosidase and AR inhibition. Among raw corns, the purple type had the highest total phenolic content and AR inhibitory activity, whereas yellow showed elevated inhibitory α-glucosidase activity and moderate ACE inhibition suggesting that non-phenolic compounds may be involved.
The antioxidant activity coupled with the antidiabetic potential, suggest that corn could be used in prevention and management of diabetes and its complications. Continuing work is important in order to identify the compounds that present the bioactivities studied which are beneficial to the human health and the effect of the processing of raw corn in masa, tortilla or chips on residual bioactivities
Recibido: 22-07-2017
Aceptado: 02-10-2017