Buriti pulp flour (BPF) contains significant levels of antioxidants. This study evaluated the effect of BPF on biomarkers of oxidative damage in the liver, heart, and pancreas of diabetic rats. The chemical composition, antioxidant capacity, and polyphenol content of BPF were determined. Thirty-six female Fisher rats were divided into four groups: control (C); control + BPF (CB); diabetic (D); diabetic + BPF (DB). Diabetes was induced by treatment with streptozotocin. Thirty days after the induction of diabetes, glucose, total cholesterol and triacylglycerides serum levels, aminotransferase and paraoxonase activities were evaluated. Oxidative damage to lipids and proteins was assessed through thiobarbituric acid reactive substances (TBARS) and protein carbonyl analyses, respectively. Histopathological analyses were also performed. BPF contained high concentrations of phenolic compounds, lipids, and fibers, and exhibited a high capacity to neutralize the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical. Diabetes was evidenced by equivalent high levels of glucose in plasma from rats in the D and DB groups. Diabetic rats in both groups also presented the same increased activity of aminotransferases. Protein carbonyl levels were increased in liver, heart, and pancreas in the D compared with C group. Although treatment with BPF did not result in any histopathological alterations, it reduced significantly the levels of TBARS in the heart and protein carbonyls in the liver and heart. No effect on blood glucose and tissue histology was observed following treatment with BPF. However, BPF diminished oxidative damage in liver and heart, indicating a possible antioxidant potential in vivo, in addition to in vitro.
Key words: Diabetes, oxidative stress, antioxidant, buriti.
La harina de pulpa buriti (BPF) contiene niveles significativos de antioxidantes. Este estudio evaluó el efecto del BPF en biomarcadores de daño oxidativo en el hígado, el corazón y el páncreas de ratas diabéticas. Se determino la composición química, la capacidad antioxidante y el contenido de polifenoles del BPF. Treinta y seis ratas Fisher fueron divididas en cuatro grupos: Control (C); Control + BPF (CB); Diabético (D); Diabético + BPF (DB). La diabetes fue inducida por tratamiento con estreptozotocina. Treinta dias después de la inducción de la diabetes, se evaluaron los niveles séricos de glucosa, colesterol total y triacilglicéridos, y las actividades de aminotransferasa y paraoxonasa. El daño oxidativo a lípidos y proteínas se evaluó a través de sustancias reactivas al ácido tiobarbitúrico (TBARS) y análisis de proteínas carboniladas respectivamente. También se realizaron análisis histopatológicos. El BPF contenía altas concentraciones de compuestos fenólicos, lípidos y fibras, y exhibía una alta capacidad para neutralizar el radical 2,2-difenil-1-picrilhidracil (DPPH). La diabetes se evidenció por altos niveles de glucosa en plasma de ratas en los grupos D y DB. Las ratas diabéticas en ambos grupos también presentaron la misma actividad aumentada de las aminotransferasas. Los niveles de proteínas carboniladas se incrementaron en el hígado, el corazón y el páncreas en el grupo D en comparación con el C. Aunque el tratamiento con BPF no dio lugar a alteraciones histopatológicas, redujo significativamente los niveles de TBARS en el corazón y las proteínas carboniladas en el hígado y el corazón. No se observo ningún efecto sobre la glucosa en la sangre y la histología de tejidos después del tratamiento con BPF. Sin embargo, el BPF disminuyó el daño oxidativo en el hígado y el corazón, lo que indica un posible potencial antioxidante in vivo, además de in vitro.
Palabras clave: Diabetes, estrés oxidativo, antioxidante, buriti.
https://doi.org/10.37527/2018.68.1.006
Diabetes mellitus (DM) is a metabolic disorder of multiple etiologies that is characterized by a state of chronic hyperglycemia deriving from abnormalities in the secretion and/or action of insulin. These abnormalities result in disturbances in the metabolism of carbohydrates, lipids, and proteins. Type 1 DM results from the attack of T cells on pancreatic beta cells. Type 2 DM has a complex pathogenesis and, in general, results from resistance to insulin at multiple levels and a relative deficiency in insulin secretion (1). Type 2 DM is one of the most important global public health problems; its prevalence has been growing each year.
Progression of DM results in pathologic alterations and complications that involve several tissues including liver, kidney, and retina, the cardiovascular system, and the central and peripheral nervous systems. Diabetic complications compromise the productivity, quality of life, and survival rates of affected individuals, besides the high cost for treatment (2). Therefore, the search for additional and less expensive therapies that are able to minimize these risks is fundamental.
The development of DM complications is, among other factors, closely related to an increase of oxidative stress. Alterations in endogenous antioxidant defense systems associated with increases in the production of reactive oxygen species (ROS) play a fundamental role in the development of tissue damage that eventually evolves to the late complications of DM (3).
Interventions designed to reduce the risk of diabetic complications have been increasing. A therapy of growing interest is the use of antioxidants. Foods that contain substances considered biologically active, such as carotenoids, and vitamins C and E, are known as functional foods when associated with an improvement in an individual’s general health and well-being, or with a reduction of the risk of diseases (4). Thus, interest in the consumption of native fruits with functional activity has been increasing and research about these foods has become very important due to their contribution to public health and the economy.
Buritizeiro (Mauritia flexuosa L.f.) is a palm tree belonging to the Palmae or Arecaceae family and is found in the Amazon and Cerrado Brazilian biomes. Its fruit, the buriti, has significant socio-economic potential. The main components of the buriti are the pulp and derived products such as oils, nectars, sweets, fermented drinks, and seeds. Buriti pulp contains considerable amounts of antioxidants including carotenoids, polyphenols, and ascorbic acid, thus presenting the potential to be used in the prevention of diseases caused by oxidative stress. Its lipid fraction is composed primarily of tocopherols and oils, predominantly oleic and palmitic acids, which help prevent cardiovascular diseases (5). Due to this composition, this fruit has received the attention of numerous researchers. Studies show that the buriti minimizes some metabolic changes related to iron overload (6), and improves the lipid profile, serum tocopherol, and retinol status in healthy young rats (7).
Despite these data on the potential benefits of the buriti, there are few studies describing its in vivo functional activities and its effect on chronic diseases such as DM. In this context, and considering that buriti pulp has a high concentration of monounsaturated fatty acids and antioxidants, the present study was designed to evaluate its effect on biomarkers of oxidative stress in the liver, heart, and pancreas of streptozotocin (STZ)-induced diabetic rats. In this way, this research will provide the scientific evidence that buriti pulp can be a secure food option for diabetic, as a part of a healthy diet.
Centesimal composition, total phenolic content and in vitro antioxidant activity Buriti pulp flour (BPF) was acquired from the local market in Belo Horizonte, Minas Gerais, Brazil, and was prepared from dehydrated pulp of buriti and without preservatives.
The determinations of residual moisture (105°C), proteins, lipids, ashes and fiber in the buriti flour were analyzed in sample triplicates, according to Association of Official Analytical Chemists methods (8).
The total phenolic content was determined according to the Folin-Ciocalteu method described by George et al (9). Gallic acid was used as standard and the total polyphenols were expressed in milligrams of equivalent of gallic acid (GAE) by 100g of flour.
The antioxidant capacity of the BPF was determined by Brand-Williams et al (10). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2- carboxylic acid) as standard antioxidant and result was expressed in μM equivalent of Trolox (TEAC).
Animals and experimental design. Thirty-six female Fischer rats, weighing around 190 g, were used at 80 days of age. The rats were obtained from the Experimental Nutrition Laboratory of the Federal University of Ouro Preto (UFOP). The animal use protocol was approved by the Committee on Ethics in the Use of Animals UFOP (Protocol: 2011/48).
Rats were divided into four groups according to the treatment received: control (C), control buriti (CB), diabetes (D), and diabetes buriti (DB). Groups C and D received the standard AIN-93M diet (11) and groups CB and DB received the standard diet containing 2% buriti pulp flour (BPF). The diets were prepared in the Experimental Nutrition Laboratory, packaged in plastic bags, and stored at -20ºC.
Initially, rats belonging to the diabetic groups received an intraperitoneal injection of STZ (35 mg/kg in 0.2 mL of 0.1 M citrate buffer, pH 4.5) after 12 hours of fasting. Control rats received an injection of sodium citrate buffer at the same time. Rats were considered diabetic when plasma glucose levels during fasting exceeded 250 mg/ dL. On the 25th day after STZ treatment, the rats were subjected to an oral glucose tolerance test (OGTT).
During the experiment, all rats were weighed weekly, kept in an environment controlled for temperature, humidity, ventilation, and received water ad libitum. After 30 days of control or BPF treatment, the rats were fasted for 12 hours, anesthetized with isoflurane, and euthanized. Blood was collected to obtain the serum and plasma. The liver, heart, and pancreas were removed, immersed in liquid nitrogen, and immediately stored at -80°C for future analyses.
Biochemical parameters in serum. The biochemical measurements of glucose, total cholesterol, triacylglycerides, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using commercial kits Labtest Diagnóstica S.A. (Lagoa Santa, MG, Brazil) according to manufacturer’s instructions.
The determination of paraoxonase (PON) enzyme activity, using paraoxon or phenylacetate as substrate, was determined as described by Beltowski et al (12).
Markers of oxidative damage. Protein carbonyl was performed according to Levine et al (13). Initially, a fragment of tissue was homogenized in buffer phosphate 50 mM (pH 6.7), the supernatant removed and used in later procedures. Next, the samples were precipitated with trichloroacetic acid (TCA) 10% and after centrifugation at 5000g for 10 minutes at 4ºC, the supernatant was discarded. After centrifugation, the precipitate was treated with DNPH 10 mM in HCl 2M, incubated in the dark for 30 min and then treated with TCA 10%. After centrifuging, the precipitate was washed twice with ethanol/ethyl acetate (1:1) and dissolved in SDS 6%. Absorbance was determined at 370 nm. The results were expressed in nmol of DNPH incorporated/mg of protein. The content of DNPH incorporated was calculated using the molar absorption coefficient of DNPH (22000 M-1cm-1).
In order to determine the lipid peroxidation, the concentration of thiobarbituric acid reactive substances (TBARS) was assessed according to the method of Buege & Aust (14). This method is based upon the capacity of the thiobarbituric acid (TBA) to bind to oxidized lipids. Primarily, a fragment of the tissue was homogenized with buffer Tris HCl 20 mM (pH 7.4), and then centrifuged for 10 minutes at 4ºC. Then, 500 μL of the supernatant of each tissue were mixed with TCA (28% p/v in HCl 0.25 M), TBA (1% in acetic acid 0.25 M) and BHT (125mM in ethanol), heated for 15 minutes to 95ºC and then put in an ice bath. The precipitate was removed by centrifugation at 13,000g for 10 minutes at 4ºC, and the absorbance of the supernatant was determined at 535 nm. The levels of TBARs were calculated using the coefficient of molar extinction of the MDA (154000 M-1cm-1).
The total protein content was determined according to the methodology described by Lowry et al (15), using bovine serum albumin (BSA) as standard.
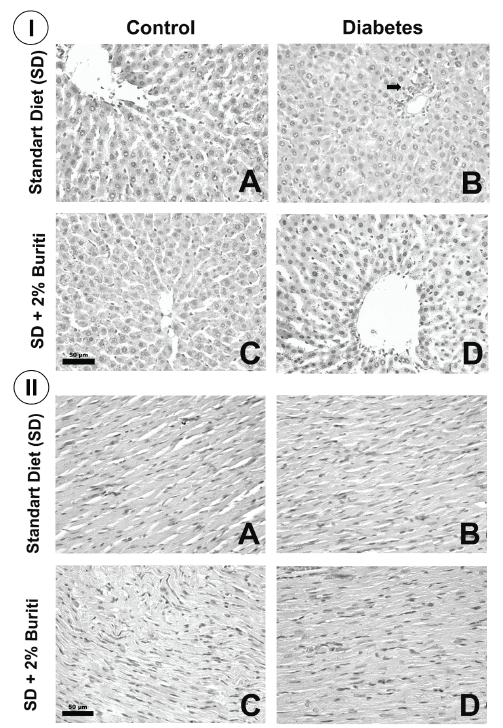
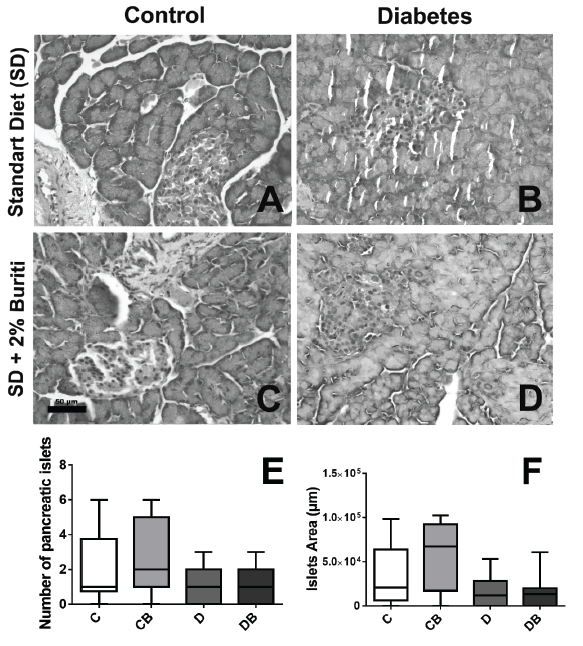
Histopathological analysis of liver, heart and pancreas. Fragments of the liver, heart and pancreas were fixed in buffered formalin at 4%. Later, these tissues were processed in an increasing series of alcohols and immersed in paraffin. Tissue sections (4 μm) were cut in microtome (Leica Germany) and mounted on microscope slides. The slides were then stained with Hematoxylin and Eosin (H&E) and photographed at 40x magnification (Leica Application Suite, Germany).
Statistical analyses. The data were submitted to normality analysis through the Kolmogorov- Smirnov test. Data with normal distribution were submitted to analysis by Student’s t-test and presented as mean ± standard error. Data without normal distribution were submitted to non parametric test Mann-Whitney and presented as median and percentiles 25 and 75. Differences were considered significant for p<0.05. All analyses were realized with the software GraphPadPrism, version 5.00 for Windows® (San Diego, California, USA).
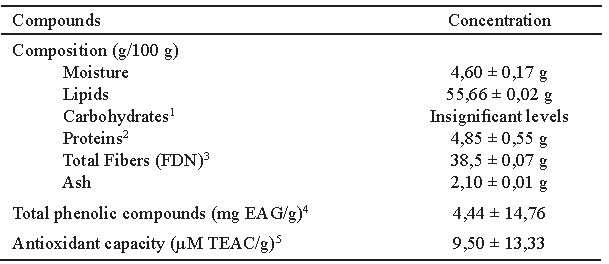
BPF contains more that 50% lipids and 38.3% insoluble fibers (cellulose, hemicellulose, and lignin). BPF also contains phenolic compounds (4.44 ± 14.76 mg EAG/g) and a high in vitro antioxidant capacity (9.5 ± 13.33 μM TEAC/g) (Table 1).
OGTT. To assess the glycemic profile of the groups studied, the OGTT was performed on the 25th day of the experiment. There was no difference between the control groups (C and CB) at all times analyzed. However, plasma glucose levels of the diabetic groups (D and DB) were significantly higher at all times compared to the control groups, having the glycemic peak at 30 minutes. No difference between D and DB groups was found.
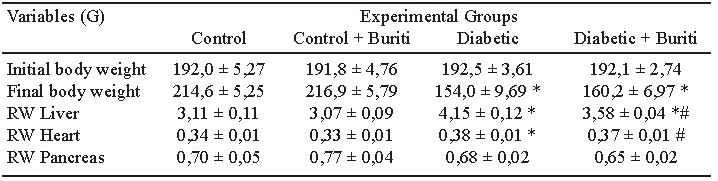
Body composition and biochemical parameters. Animals from groups D and DB presented final body weights significantly lower than the control groups. Furthermore, the relative weight of the liver and heart increased in group D compared to group C rats. In contrast, animals from the DB group decreased the relative weight of both organs when compared to D group. There was no difference in the relative weight of the pancreas between groups (Table 2).
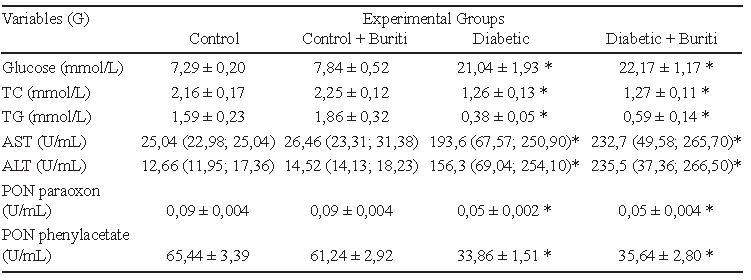
Plasma glucose levels of the diabetic groups were significantly higher than the control groups, confirming the efficiency of inducing diabetes with STZ. Supplementation with 2% BPF had no effect on the glycemic profile of animals in the CB compared to the C group, nor in the DB compared to the D group. Moreover, the animals of both diabetic groups showed significantly lower levels of total cholesterol and triacylglycerides, and reduced enzyme paraoxonase activity using paraoxon or phenylacetate as substrate. Animals in the D and DB groups exhibited an increase in the activities of the serum enzymes ALT and AST (Table 3).
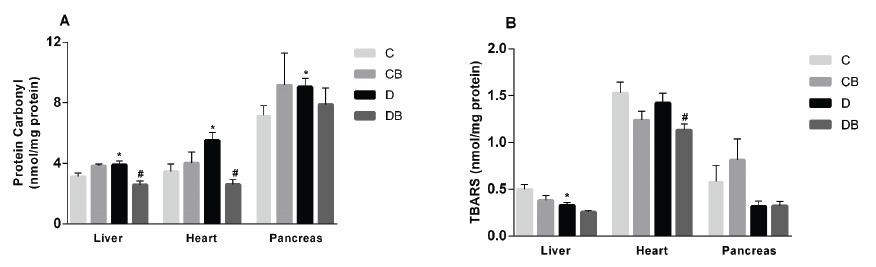
Oxidative damage markers and histopathological analyses. The effect of dietary supplementation with BPF on biomarkers of oxidative stress, and the histopathology of the liver, heart, and pancreas, were assessed. Increased oxidative stress in diabetic rats was evidenced by the high levels of protein carbonyls in liver, heart, and pancreas compared to the control group. The addition of 2% BPF to the diet caused a significant reduction of the protein carbonyl levels in liver and heart of diabetic rats, compared to D group (Figure 1A). TBARS levels were significantly lower in the D compared to the C group in liver. However, no change was induced by buriti treatment, although the DB group did show reduced levels of this biomarker in heart. In pancreas, there were no significant differences among the experimental groups (Figure 1B).
Histopathological analyses of liver, heart, and pancreas showed that there were no significant alterations among the experimental groups, although microscopic analysis of the pancreas indicated a tendency to reduce the number and area of pancreatic islets in groups D and DB (Figures 2 and 3).
Currently, there is growing interest in the use of natural antioxidants as a strategy to reduce the complications of DM caused by the increase of oxidative stress. In this context, the availability of foods rich in antioxidant compounds has become an option for treatment and risk reduction of various diseases associated with oxidative stress. Our results show that BPF has antioxidant potential because it exhibits a high capacity to neutralize the DPPH radical in vitro, has a considerable content of total polyphenols, and reduces the oxidation of proteins in liver and heart of diabetic rats.
Our data revealed a greater content of lipids and insoluble fibers in BPF compared to the study of Melo et al. (16) who used fresh pulp. The higher values of these compounds that we found may arise from the loss of moisture during the thermal processing that was necessary to produce the flour. Our result for total phenolic content was similar to the value reported by Cândido et al. (17) (435 mg AGE 100 g-1) but was higher than that found by Manhães et al. (5) and Romero et al. (18). Climatic factors, the time of maturation, species, geographic origin, growth stage, harvest conditions, and storage of the fruits can influence the content of total polyphenols and antioxidant capacity (17).
Other studies show that buriti pulp contains high concentrations of lipids, mainly unsaturated fatty acids and carotenoids (5, 16). Thus, considering the presence of polyphenols, carotenoids, and ascorbic acid, we used the DPPH method to test the antioxidant activity of BPF. This assay revealed that BPF had a high in vitro antioxidant capacity because it was able to neutralize the DPPH radical, even at low concentrations, corroborating the study of Romero et al. (18).
We observed that STZ-induced diabetic rats had glycemia significantly higher than the control rats. Moreover, the diabetic animals presented lower body weights at the end of the experiment, which was also observed by Guerra et al. (19). This weight loss and the reduced levels of total cholesterol and triacylglycerides is a characteristic of the diabetic condition and is mainly due to the catabolic state derived from the greater action of counter regulatory hormones. Moreover, as insulin stimulates the synthesis of cholesterol and triacylglycerols in the liver through the activation of the enzymes HMG-CoA reductase and ACC respectively, a reduction in the production of this hormone may lead to a decrease in the blood concentrations of these metabolites (20). We also observed an increase in the relative weight of the liver and in the activity of the enzymes AST and ALT, showing a possible hepatic dysfunction. The enzyme paraoxonase (PON) has antioxidant capacity and circulates in plasma associated with HDL protecting lipoproteins from the action of free radicals, mainly against the oxidation of LDL. Thus, a reduction in their blood levels may favor LDL oxidation and, as a consequence, the development of atherosclerosis, a frequent condition in diabetic patients. There are two distinct methods of dosing PON activity. In our study, we found a significant reduction in PON activity in diabetic rats at both dosages. However, the treatment with 2% BPF was not able to reverse this situation.
Excess of free radicals due to an imbalance between the production of ROS and endogenous antioxidant capacity promote the oxidation of biomolecules with consequent loss of their biological functions and/or homeostatic imbalance. In this study, we evaluated the effect of adding BPF to the diet on oxidative damage to cellular proteins and lipids. Carbonyl groups are present in all proteins, form the basis of their structural integrity, and influence their capacity to function and interact with other molecules. However, excessive introduction of new carbonyl groups can result in altered or disrupted protein functions (21). Most oxidative damage markers reflect the attack of free radicals on polyunsaturated fatty acids with resultant lipid peroxidation. Lipid peroxidation is initiated by the reaction of a reactive species with an unsaturated fatty acid and is propagated by the peroxyl radical. Malondialdehyde is the most abundant aldehyde resulting from lipid peroxidation (22) and is often measured indirectly as TBARS as an index of this process.
In the liver, oxidative stress plays a specific role in the pathogenesis of fibrosis and hepatic diseases. Oxidative stress in DM is one of the major factors triggering the complications associated with this disease. We found high levels of protein carbonyls in the diabetic groups, as has been found by others (19). BPF was able to reduce significantly the protein carbonyl levels in liver and heart, suggesting a possible reduction of oxidative damage to proteins in these tissues in DB group. In the present study, a reduction of TBARS levels in the hepatic tissue of diabetic rats fed with BPF was observed. Although it still remains to be clarified, the reduced TBARS level in the diabetic rats may have been due to the deficiency of insulin provoked by the model, resulting in changes in the metabolism of glucose and lipids. A deficiency of insulin leads to an increase in lipid catabolism and, thus, the diabetic rats may contain a lesser amount of lipids reducing the possibility of lipid peroxidation. In contrast, the study of Romero et al. (18) found that the intake of buriti pulp did not affect the concentrations of TBARS and catalase in liver, but increased the levels of non-protein sulfhydryl groups in male rats, demonstrating a greater stimulation of antioxidant activity by including this fruit in the diet.
Reports from the Framingham and other studies have shown that DM represents a strong risk factor related to cardiovascular disease. Shao et al. (23) found a correlation between protein carbonyls and heart dysfunction due to the loss of activity of proteins that participate in the contraction and relaxation of heart muscle. Our results showed that there was an increase of oxidative stress in the cardiac tissue of diabetic rats, revealed by the increase in the concentration of protein carbonyls. The addition of BPF reduced the concentrations of protein carbonyls and TBARS in diabetic rats, suggesting that the buriti had an antioxidant effect in this organ.
The overproduction of ROS induced by hyperglycemia during diabetes can cause cellular damage to the pancreas by creating a redox imbalance. Pancreatic beta cells have poor antioxidant defenses and inefficiently repair oxidatively damaged DNA (24). Thus, several strategies to boost the antioxidant defenses of islets have been evaluated. The implementation of antioxidant therapy may help strengthen the defense status of the cells in this organ. The results of our study revealed an increase in protein carbonyl levels in the pancreas of diabetic rats, but no change in TBARS levels in this organ among the different groups.¬ Although another study reported that antioxidants like vitamins C and E, curcumin, quercetin, and resveratrol are efficient in rescuing islet cells from damage caused by free radicals (25), the amount of antioxidants in BPF in this study may have been insufficient to reverse the oxidative damage to proteins following treatment of rats with STZ.
The histopathological analyses did not reveal any alterations in the liver, heart, and pancreas of diabetic rats. This shows that, despite the increased oxidative stress, there were no morphological changes in these tissues. This result may have occurred because the serious clinical condition induced by STZ resulted in insufficient time for histological alterations to develop, as well as a short period of treatment with BPF. Moreover, due involvement of multiple pathways in the increase ROS formation in diabetes, a dietary antioxidant may not be sufficient to reverse the adverse effects of oxidative stress.
Overall, our results indicated that supplementation of the diet with BPF in diabetic rats promoted a reduction in oxidative damage to proteins in the liver and heart, without interfering with the weight, glycemic profile, aminotransferase activities, total cholesterol and triacylglycerides levels, and histological profile of the analyzed tissues. Then, although BPF did not present conclusive results in diabetic rats, we believe that buriti pulp has antioxidant potential in vitro and in vivo, but additional research should be performed using different experimental models of chronic diseases to gain a better understanding of the effects of this fruit and its potential contribution to public health.
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Pesquisa de Minas Gerais (Fapemig, Minas Gerais, Brazil).
Disclosure: no potential conflict of interest relevant to this article was reported.
Recibido: 25-07-2017
Aceptado: 18-11-2017