Lemon balm (Melissa officinalis) is a plant in the family of Lamiaceae. In Mexican traditional medicine it is used to alleviate gastrointestinal and hepatic problems. Studies carried out mainly in ethanolic and methanolic extracts, have revealed the presence of diverse compounds to which those medicinal properties are attributed. The objective of this research work was to extract in aqueous solution the antioxidants present in lemon balm and identify them through HPLC-MS. A Box-Behnken design was applied to determine the physical conditions of antioxidant extraction, where the analyzed variables were time, temperature and sample quantity. The antioxidant activity was determined through methodologies of DPPH*, FRAP and total phenolics. The aqueous solution with the highest antioxidant activity was analyzed through HPLC-MS. The results showed that the interaction temperature-time has a positive influence on the liberation of antioxidants. The best condition for a conventional extraction of antioxidants was 90°C, 15 min and 2 g of sample. Higher correlations were observed at r2>0.6 between determined antioxidant activity by DPPH* (or FRAP) vs Total phenolics; this would indicate that such activity could be attributed to phenolic compounds whose presence was confirmed through an analysis by HPLC-MS.
Palabras clave: Lemon balm, Melissa officinalis, antioxidants, HPLC-MS, DPPH*, FRAP, Total phenolics.
El toronjil (Melissa officinalis) es una planta de la familia Lamiaceae. En la medicina tradicional mexicana es utilizado para aliviar problemas gastrointestinales y hepáticos. Algunos estudios realizados con extractos etanólicos y metanólicos de dicha planta, han revelado la presencia de diversos compuestos a los que se les atribuye sus propiedades medicinales. El objetivo de esta investigación fue extraer en solución acuosa los antioxidantes presentes en el toronjil e identificarlos a través de HPLC-MS. Para ello, se aplicó un diseño de experimentos Box-Behnken a fin de determinar las condiciones físicas de extracción de antioxidantes; las variables analizadas fueron tiempo, temperatura y cantidad de muestra. La actividad antioxidante fue determinada a través de las metodologías de DPPH*, FRAP y fenoles totales. El extracto acuoso con la mayor actividad antioxidante fue analizado mediante HPLCMS. Los resultados mostraron que la interacción tiempotemperatura tuvo una influencia positiva en la liberación de antioxidantes. La mejor condición para la extracción de antioxidantes presentes en el toronjil fue 90°C, 15 min y 2 g de muestra. Correlaciones superiores a r2>0.6 fueron determinadas entre la actividad antioxidante medida por DPPH* (o FRAP) vs Fenoles totales; esto podría indicar que la actividad antioxidante encontrada podría atribuirse a compuestos de tipo fenólico cuya presencia fue confirmada por el análisis en HPLC-MS.
Key words: Toronjil, Melissa officinalis, antioxidantes, HPLC-MS, DPPH*, FRAP,
https://www.doi.org/10.37527/2018.68.3.009
The therapeutic effects that plants offer are very important for human health. According to the World Health Organization around 60% of the population is still treating health problems with traditional remedies (1). At this respect, more than 20,000 species of plants have been researched and are used to treat several pains or diseases in traditional medicine and as flavoring or seasoning species for food preservation or storage (2).
One of the most common uses of plants in traditional medicine is the preparation of infusions with their leaves, flowers, fruits and/or peels through an extraction process with hot or cold water during certain time (3). These beverages are widely consumed around the world and are considered as one of the main reservoirs of natural antioxidants. The antioxidant capacity of some infusions is attributed to the presence of phenolic compounds (3) considered bioactive due to properties such as anti-inflammatory, antimicrobial, analgesic, neuroprotection, among others (4).
Lemon balm (melisa or balm) is a plant in the Lamiaceae family. Its dry leaves are widely used in Mexican traditional medicine to treat gastrointestinal problems, biliary and liver diseases, mental disorders, diseases of the central nervous system, respiratory and cardiovascular problems, and several types of cancer, etc. (5).
Several studies have been carried out to extract and identify the compounds responsible for the antioxidant activity in lemon balm, using mainly using organic matrices (propanol, methanol and ethanol). Some of the identified chemicals are bioactive substances such as eugenol, citral, caffeic acid derivatives (rosmarinic acid), flavonoids (cynaroside, cosmocin, rhamnocitrin, isoquercitrin), phenolic acids (carnosic acid), and triterpene acids (ursolic and oleanolic acid) (6). In literature, there is few information about the use of water as the only agent for the extraction of antioxidant properties in lemon balm and their identification.
The objective of this research work was to establish the physical conditions of extraction in aqueous solution of antioxidant compounds present in lemon balm. For that, it was applied a Box-Behnken experimental design considering the variables temperature, time and sample quantity. In addition, the compounds with antioxidant activity present in the obtained extracts were identified by HPLC-MS.
Approximately 2 kg of the whole plant of lemon balm were bought in a local market located in Pachuca, Hidalgo (Mexico). The whole sample was exposed to the sun at a room temperature, during 15 days with the purpose of decreasing the content of water and avoiding fungus growth. Once it was dry, the different parts of the plant were separated (leaf, stem and stem with leaves) and preliminary tests were performed to determine which had the highest antioxidant activity. After several tests, the selected part was the leaf.
Physical conditions for the aqueous extraction of antioxidant compounds from lemon balm through a Box-Behnken design.
To obtain the aqueous extract of lemon balm with the highest antioxidant activity (AA) and to be able to identify the compounds responsible for such activity, a Box-Behnken central fractional factorial design was applied. The control factors and the selected levels for the extraction process are shown in Table 1. Those conditions were selected based on the normal conditions on which an infusion is prepared and according to some similar data reported in literature (7).

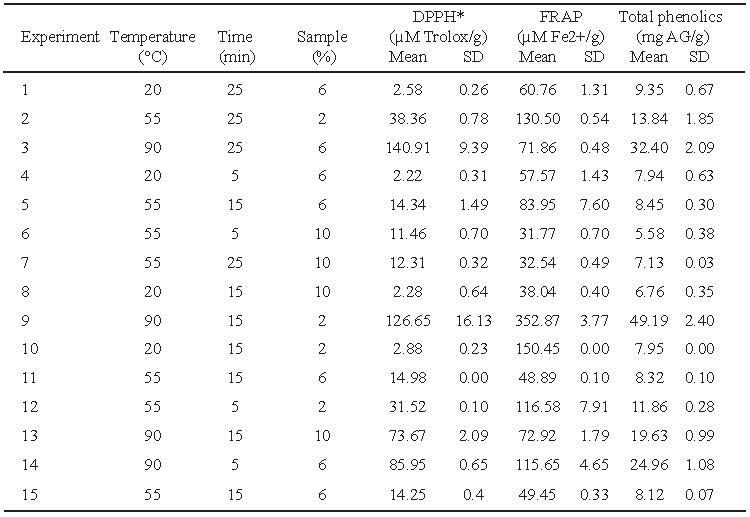
From the performed experimental design, 15 experiments were conducted in triplicate following the conditions described by Ramírez et al. (7). The antioxidant activity of the aqueous extracts was determined through DPPH* free radical assay, Ferric Reducing Antioxidant Power method (FRAP) and the content of total phenolics was determined by Folin & Ciocalteu’s method. A conventional solid-liquid extraction was performed using a jacketed beaker (Schott Duran®), a stirring heater (Nuova Sarrer-Barnstead Thermolyne®) at 600 rpm, a recirculating water bath (VWR®) and distilled water, as extraction solvent.
The method, proposed by Brand-Williams et al. in 1995, was used with some modifications. A calibration curve was prepared [0 a 33 μM], from a standard solution of Trolox 1.0 mM en MeOH. To each standard solution, 2.9 mL of DPPH* (2,2-diphenyl-1-picrylhydrazyl) 0.1 mM in MeOH, were added. All standard solutions were taken to a final volume of 3 mL with MeOH. Together with the standards, a control sample was prepared containing only 0.1 mL of MeOH and 2.9 mL of DPPH*. Standards were left to react in darkness for 50 minutes and the absorbance was measured at 515 nm, using methanol as blank. The AA of the aqueous extracts was measured using the same procedure than for the calibration curve, replacing the Trolox solution with 100 μL of each extract of lemon balm. All determinations were made in triplicate.
Finally, the calibration curve was made with [Trolox] vs % DPPH* remnant data, as of the absorbance obtained values for the control sample and for each standard. The percentage of DPPH*remnant was calculated.
This analysis was carried out using the modified Benzie and Strain’s FRAP method. The reactive FRAP was prepared from an acetate buffer (300.0 mM to pH 3.6), ferric chloride hexahydrated (20.0 mM) and TPTZ (4,6-tripryridyl-s-triazine): 10.0 mM, prepared in HCl 40.0 mM. The three solutions were mixed in proportions of 10:1:1 (v/v/v).
A calibration curve was formed, [0 to 100 mM], from a standard solution of ferric chloride tetrahydrated in HCl 40.0 mM. To each standard solution of the curve 1 mL of FRAP reactive was added and it was taken to a final volume of 10.0 mL with distilled water. All the solutions were incubated at 37 °C for 4 minutes and its absorbance was measured at 593 nm using a blank containing only FRAP reactive. The AA of the aqueous extracts was measured using the same procedure used for the calibration curve, replacing the ferric chloride tetrahydrated with 250.0 μL of each extract of lemon balm. Every determination was made in triplicate.
A calibration curve was formed to estimate total phenolics at a concentration interval of 0 to 15.0 mg/L, as of a standard solution of gallic acid (GA) 1000.0 mg/L. The corresponding volume was taken from each standard, 2.0 mL of Na2CO3 were added to 7.5%, 2.0 mL of Folin & Ciocalteu’s reactive and it was diluted to 10.0 mL with distilled water. The absorbance readings were made at 760 nm. To determine phenols in aqueous extracts of lemon balm, the same procedure was followed as for the calibration curve, replacing GA with 1.0 mL of each sample. The determinations were made in triplicate.
Identification of chemical compounds responsible for AA in the lemon balm aqueous extract was performed by using an HPLCMS equipment (Agilent Technologies series 1200) with a Q-TOF spectrometer (Agilent Technologies, mod. 6530A). The column used was a Phenomenex Kinetex Biphenyl (50 x 2.1 mm, 2.6 μm). The mobile gradient phases used were phase A: water with 0.1% formic acid and phase B: methanol and 0.1% formic acid with a flow rate of 0.4 mL/min.
Analysis of results. From the data obtained using the Minitab 17 program, the polynomial experimental designs were achieved; contour plots and response surface graphs were formed. With the purpose of determining the influence of the analyzed variables (temperature, time and sample quantity) on the liberation of antioxidant compounds from aqueous extracts of lemon balm, a comparative analysis of the response graphs on the different treatments was performed. Finally, the correlation between the concentration of the antioxidant compounds measured by DPPH* and FRAP methods, and the content of total phenolics in the aqueous extracts of lemon balm was established. To validate the experimental model, a prediction interval was obtained for each assay.
Preliminary assays performed to lemon balm leaves, stems and stems with leaves showed that the highest concentrations of compounds with antioxidant capacity were extracted from leaves (data non-showed), that is why in this study it was decided to work only with this part of the plant.
The polynomial obtained for the antioxidant activity determined by DPPH*, FRAP and total phenolics are presented in Table 2. The variables temperature and time showed a positive effect, while the variable sample quantity showed a negative effect. The data analysis showed that the interaction temperature-time has an important influence in the liberation of compounds with antioxidant activity present in the leaf of lemon balm.
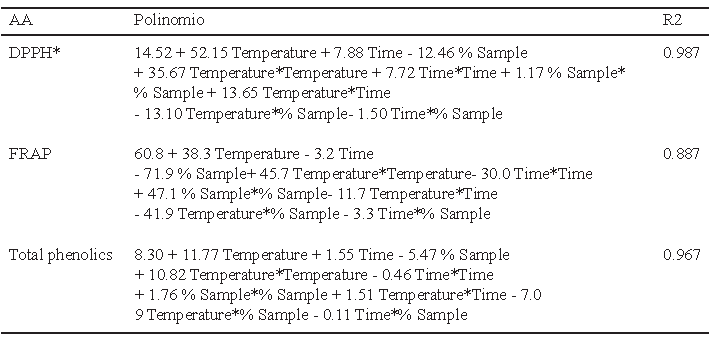
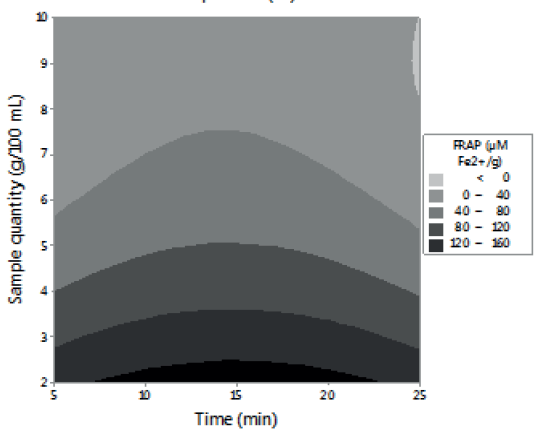
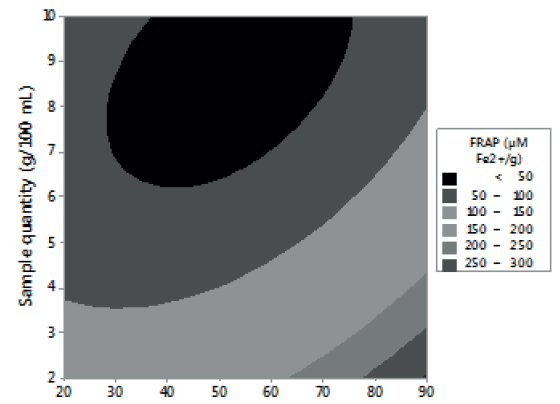
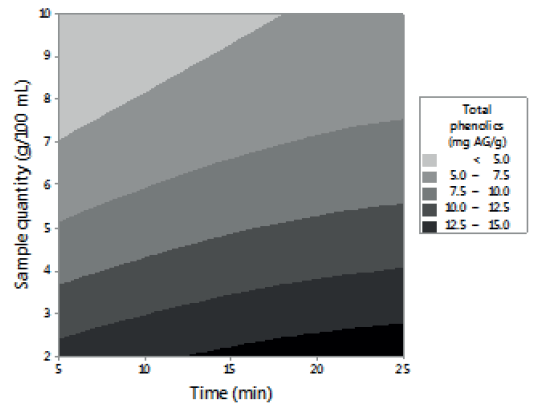
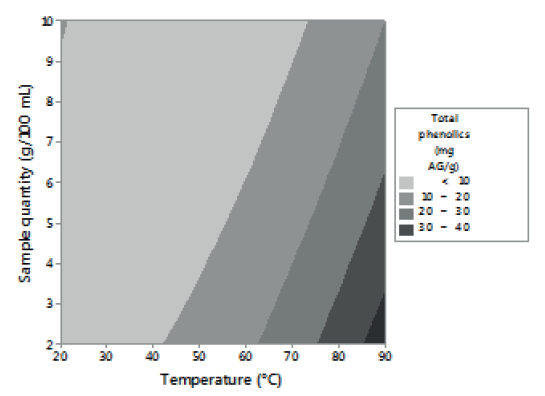
Figures 1, 2 and 3 allow visualizing the effect of each response in terms of the analyzed variables (time, temperature and % of the sample). In Figure 1 it is observed the interaction between the analyzed variables (temperature and time; % of sample and temperature; % of sample and time) and the effect on the antioxidant activity measured by DPPH*. Figure 2 shows the values of antioxidant capacity of aqueous extracts of lemon balm measured by FRAP method. The values of total phenolics for aqueous extracts of lemon balm are presented in Figure 3.
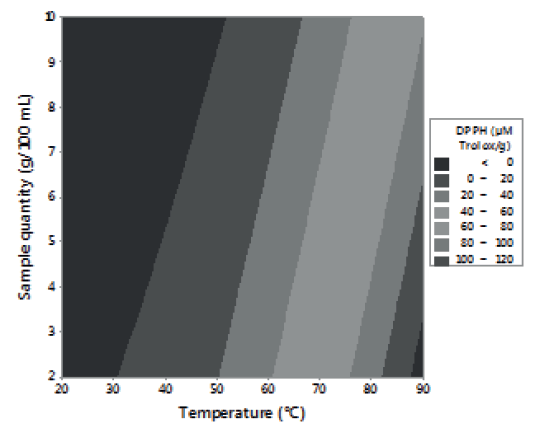
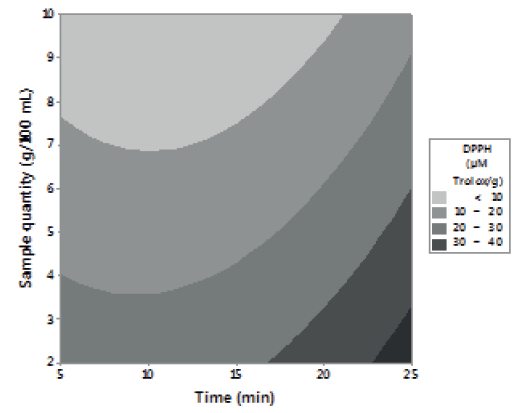
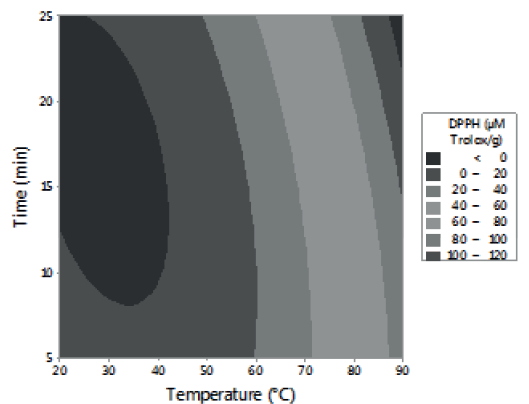
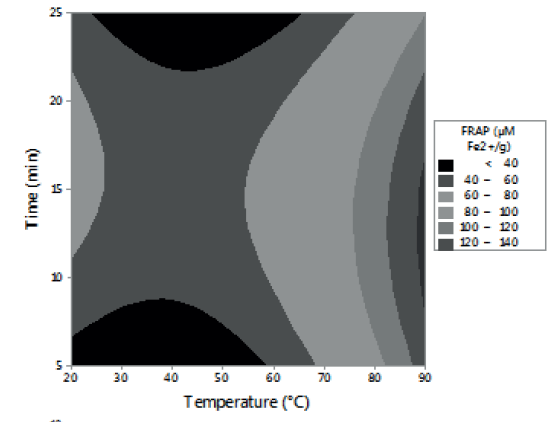
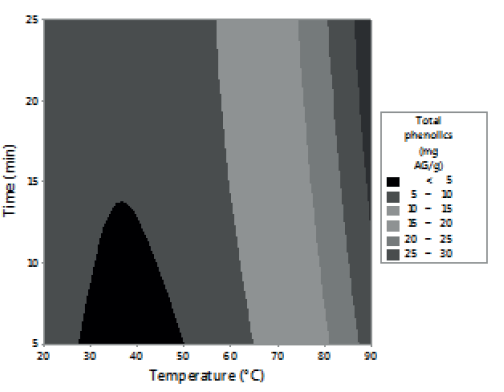
The AA measured by DPPH* technique was variable, obtaining values from 2.28 to 140.91 μM Trolox/g. The highest antioxidant activity was observed in the extracts prepared with 2 and 6 grams of sample at a temperature of 90°C during a time of extraction of 15 and 25 minutes, respectively.
Regarding the antioxidant capacity determined by FRAP, through this method the values obtained were in an interval of 31,77 to 352,87 μM Fe2+/g. The highest value corresponded to the extracts prepared with 2 grams of sample, extracted at 90°C during 15 minutes; these conditions coincide with the ones determined by DPPH*.
In relation to the content of total phenolics in aqueous extracts of lemon balm, these presented values from 5.58 to 49.16 mg AG/g. The highest concentration corresponded to the extract prepared at 90°C with 2 grams of sample during 15 minutes; the condition of temperature is in agreement with that determined in DPPH* and FRAP. (Table 3)
Identification of compounds in aqueous extract of lemon balm with higher antioxidant activity using HPLC-MS
Once the physical conditions (time, temperature and % of sample) for the extraction of compounds with antioxidant activity in lemon balm infusions were optimized through a Box-Behnken design, the compounds responsible of such activity were identified by HPLC-MS.
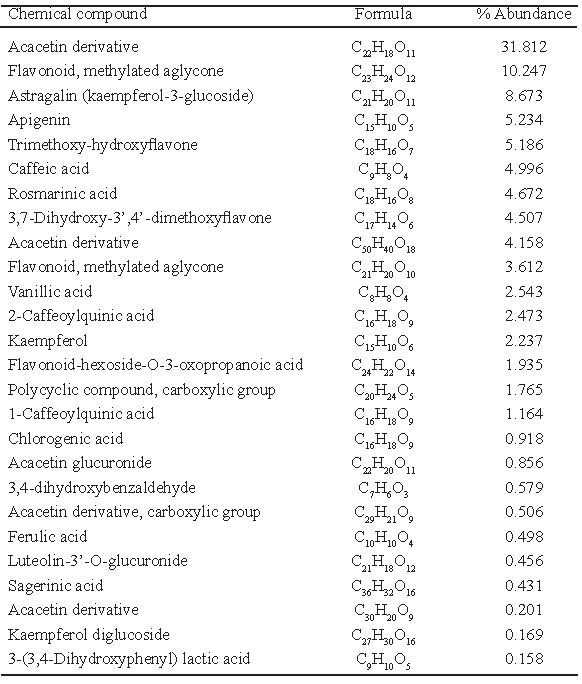
The HPLC-MS analysis of the aqueous extract of lemon balm leaves with higher antioxidant activity revealed the presence of different compounds (Table 4), mostly phenolic acids and flavonoids. Some of them were: vanillic acid (tr= 0.983 min), 1-caffeoylquinic acid (tr_ 1.44 min), 3,4-Dihydroxybenzaldehyde (tr=1.674), 2- caffeoylquinic acid (tr=2.94min), chlorogenic acid (tr=3.17 min), ferulic acid 8tr=5.932 min), luteolin -3’- O-glucuronide (tr=6.047 min) and 3- (3,4 - dihydroxiphenyl) lactic acid (tr= 6.047 min).
The data obtained of the experimental design showed that the interaction temperature-time has an important influence in the liberation of compounds with antioxidant activity present in the leaf of lemon balm. In general, it was observed that temperature had more influence on the aqueous extraction of antioxidants than the time and the percentage of sample used.
For FRAP method, the results indicate that temperature and a low percentage of sample favor the aqueous extraction of compounds with antioxidant activity (Figure 2). Temperature was the factor with the highest influence on the extraction of phenolic compounds (Figure 3), regardless of the time and the percentage of sample used. These results agree with the report of Kim et al. (8). These authors observed that, by increasing temperature, as well as contact time, the efficiency of rosmarinic acid extraction increased.
The results of AA measured in this study by DPPH* method are similar to the values reported by Wojdylo et al., (9) for extracts of lemon balm obtained by using a mixture of acetone-wateracid glacial acetic (70:28:2 w/v). However, in general, our results of AA of lemon balm are lower than those presented by several authors (10), who found values of 406.03 μM Trolox/g. This difference can be attributed mainly to the system of extraction applied. Those authors used an ultrasonic bath and methanol as extraction agent, while in our study it was used a conventional solid-liquid extraction in aqueous solution.
On the other hand, values of 389.52 μM Trolox/g for an extract of Melissa officinalis obtained by the infusion of the dry plant in water for 30 minutes have been reported (11). Although the Box-Behnken design applied in this study indicates that the temperature is an important factor in the extraction of antioxidant compounds, other factors like time, extraction solvent and the method used, have a significant influence on the antioxidant activity of the analyzed extracts. Finally, the chemical structure of the antioxidant compounds should be considered. In that regard, Bondet et al., (12) point out that the mechanism of reaction between the antioxidant and the DPPH* depends on the structural formation of the antioxidant; some compounds react quickly reducing the number of molecules of DPPH* to the same number of available OH- groups.
The highest value (352,87 uM Fe2+/g) found in this study for lemon balm by FRAP method, is higher compared to the infusion of Teucrium arduini L., other plant of the family of Lamiaceae, which had a reducing power of 136.83 μM Fe2+/g (13). In other research carried out, the authors reported 274.85 μM Fe2+/g of antioxidant activity in methanolic extracts of Salvia ringens L. (14); this value is lower than the one obtained in this work for lemon balm, despite using methanol as extraction agent.
The highest values of antioxidant activity (49.16 mg AG/g) of lemon balm determined in this study are superior to those reported by Samec et al. (13) for Peumus boldus (34.3 mg AG/g) and Soto et al. (15) for Teucrium arduini L. (23.49 mg AG/g), both infusions prepared at 90°C. The variations in the results can be due to the quantity of sample and the time of extraction used in both studies. Several studies coincide with the fact that an increase in the time of infusion favors the extraction, enhancing both the solubility of the solute, as well as the coefficient of diffusion (16).
The extracts obtained in this study for lemon balm have a higher concentration of total phenols compared to other plants studied as Agastache foeniculum (anise hyssop 27.19 mg AG/g), Lavandula angustifolia (lavender 12.44 mg AG/g) and Nepeta cataria (catmint 14.66 mg AG/g) (17). However, in other studies carried out in methanolic extracts of lemon balm leaves, higher concentrations of total phenolics were obtained Kamdem et al. (18) and Duda et al. (17). Nevertheless, it is important to point out that the extraction time in those researches was from 4 to one week. In addition, the use of methanol, in both studies favored the solubility of compounds of phenolic type. In this regard, Soto et al. (15) studied that the extraction of antioxidant compounds depends on the polarity of the solvent. For example, non-polar solvents, such as petroleum hexane and ether are used to extract tocopherols and some phenolic terpenoids, while diethyl ether and ethyl acetate are effective in extraction aglycones and phenols of low molecular weight. The solvents with the highest polarity such as ethanol and methanol may extract flavonoids and phenols of higher molecular weight. Another factor that affects the antioxidant activity in infusions is the type of process plants have been subject to, meaning if they are used as fresh plants or dehydrated plants, the part of the plant that is used, as well as if it is a complete or ground plant.
It was found a correlation of 0.8718 between the antioxidant activity measured by DPPH* vs total phenolics and of 0.6067 for the values of FRAP vs total phenolics. These could indicate that part of the antioxidant properties of lemon balm leaves can be attributed to the presence of phenolic compounds (rosmarinic acid, caffeic acid and their methyl esters), as stated by Kim et al. (8) and Caniova and Brandsteterova (19). Similar compounds have been identified as bioactive in several commonly used herbal medicine in Mexico (20).
Identification of compounds in aqueous extract of lemon balm with higher antioxidant activity using HPLC-MS
The AA in the aqueous extracts of lemon balm, determined by different methodologies (DPPH*, FRAP and Total phenolics), was correlated to the presence of phenolic compounds, as showed the correlation established by DPPH* (or FRAP) vs Total phenolics (r2>0.6). This was also demonstrated by the HPLC-MS analysis which revealed the presence of different compounds, mostly phenolic acids and flavonoids (Table 4). Caniova and Brandsteterova (19) suggested that the antioxidant activity in lemon balm is attributed to the presence of phenolic acids, mainly derived from hydroxycinnamic acids like rosmarinic acid. Kim et al. (8) also stated that the antioxidant property of lemon balm extracts is due to the presence of phenolic compounds like caffeic acid and its methyl esters. This compounds were were also identified in the present study
On the other hand, Barros et al. (21) indicated the presence of lithospermic A, salvianolic A and C acids, as well as rosmarinic acid and its dimer the sagerinic acid in infusions of lemon balm. These compounds coincide with the ones found in this research work, as well as the ones reported by Caniova and Brandsteterova (19) who found vanillic, caffeic, syringic and rosmarinic acids. Arceusz and Wesolowski (22) identified the presence of six phenolic acids (gallic, caffeic, chlorogenic ferulic, syringic and rosmarinic acids). Pereira et al. (23) state that the main compounds with antioxidant activity present in lemon balm are the gallic, chlorogenic, caffeic, ellagic acids, catechin, epicatechin, quercetin and rutin.
It has been demonstrated that the phenolic content of lemon balm varies in different regions. Samples from Bosnia and Herzegovina showed a highest concentration of rosmarinic and chlorogenic acids, compared to lemon balm from Turkey. Other compounds identified in samples from Iran and extracted with methanol are the caffeic acid; 2, eriodictiol - 7 - O - glucoside; 3, m-coumaric acid; 4, naringin; 5, hesperidin; 6, rosmarinic acid; 7, naringenin and 8, hesperetin (24).
It is important to consider that the variability of phenolic compounds depends on the polarity of the solvent during their extraction. That is why the work by Duda et al. (17) found in highest proportion of ferulic acid in samples extracted with methanol, while in other study was found rutin in ethanolic extracts of lemon balm (18).
Finally, it is important to point out that the presence and abundance of antioxidant compounds in lemon balm depend on several factors such as the variety used, the region of origin, the solvent used, and the physical conditions of extraction, mainly temperature as was demonstrated in this study.
The Box-Behnken experimental design allowed establishing the physical conditions for the extraction of antioxidant compounds of lemon balm in aqueous solution, being the temperature a determinant factor for its extraction. The correlations found between the determinations of antioxidant activity by DPPH* (or FRAP) vs total phenolics indicated the presence of compounds of phenolic type. Rosmarinic acid, caffeic acid and apigenin were confirmed by HPLC-MS as some of the most abundant compounds, although, it is important to remark the presence of other compounds in aqueous extracts of lemon balm which could have a positive effect on health.
Recibido: 07-07-2018
Aceptado: 06-11-2018