It has been proposed that the consumption of common beans (Phaseolus vulgaris L.) reduces cardiovascular risk, and prevents and controls both chronic and degenerative diseases. The aim of this study was to compare the antioxidant capacity of a bean-fiber fortified bar (BFB) versus a commercial bar (CB) in 60 Mexican men and women (18-65 years old), who were randomly distributed in two groups: BFB or CB; individuals consumed a bar a day for one month. Anthropometric data, food intake and blood samples were collected. Glucose tolerance (GTT), lipid profile (PL), and lipid peroxidation (TBARS) tests were performed; carbonyls groups in serum oxidized proteins were also measured. GTT and PL were not different between both groups in either the 15 or 30-day follow-up of bar consumption assessments. There were no significant differences in either TBARS or carbonyl concentration between groups; BFB group showed higher levels of serum lipid peroxidation in basal and fifteen days measurements; these levels decreased at the final evaluation: No differences were detected on carbonyl levels between groups. In conclusion, 30 days of fiber bean bar consumption did not alter glucose or PL levels, while, in the BFF group, oxidative stress decreased within 30 days of the consumption of the fortified bar. ALAN, 2019; 69(2): 80-88.
Key words: Barra fortificada con fibra de frijol; TBARS; carbonilos.
Se ha propuesto que el consumo de frijol común (Phaseolus vulgaris L.) reduce el riesgo cardiovascular, y previene y controla las enfermedades crónicas y degenerativas. El objetivo del presente estudio fue comparar la capacidad antioxidante de una barra fortificada con fibra de frijol (BFB) versus una barra comercial (CB) en 60 hombres y mujeres mexicanos (18-65 años de edad), quienes aleatoriamente fueron distribuidos en dos grupos: El grupo BFB y el CB que consumieron la barras fortificada con frijol y la barra comercial, respectivamente, durante un mes. Se recopilaron datos antropométricos, ingesta de alimentos y muestras de sangre. Se realizó prueba de tolerancia a la glucosa (GTT), el perfil de lípidos (PL), la peroxidación de lípidos (TBARS) y la cuantificación de carbonilos en proteínas oxidadas como pruebas de bioquímica sanguínea. GTT y PL no fueron diferentes entre ambos grupos en la evaluación de seguimiento de 15 y 30 días del consumo de la barra. No hubo diferencias significativas en los TBARS o la concentración de carbonilo entre los grupos, el grupo BFB mostró niveles más altos de peroxidación de lípidos en suero en la fase basal y a los quince días del consumo de la barra; curiosamente, estos niveles disminuyeron en la evaluación final. No se detectaron diferencias en los niveles de carbonilo entre los grupos. En conclusión, 30 días de consumo de barras de fibra de frijol no alteraron los niveles de glucosa o PL; mientras que, en el grupo BFB, el estrés oxidativo disminuyó a los 30 días del consumo de la barra fortificada. ALAN, 2019; 69(2): 80-88.
Palabras clave: Bean-fiber fortified bar; TBARS; carbonyls.
https://doi.org/10.37527/2019.69.2.002
Autor para la correspondencia: Herlinda Aguilar-Zavala, email: [email protected]
Unhealthy food habits, such as hypercaloric diets that contain large amounts of refined sugars and fat, produce systemic oxidative stress and brain damage (1). There is growing interest in the consumption of natural foods as main sources of both antioxidants and nutritional substances to reduce the oxidative stress and improve health. Many studies have demonstrated that different types of beans elicit different biological responses.
Some research have showed that black and pinto beans could be a stronger antioxidant source; the phenolic components of bean would be used as a source of antioxidants and could prevent diseases with important reactive oxygen species (ROS) production (2-4). Other studies have observed that bean consumption is helpful to prevent and control chronic and degenerative diseases, because beans reduce the glycemic stress and improve the LDL-cholesterol levels, so glycemic control could be improved through a possible insulin-sparing mechanism (5-7).
Animal models showed that bean consumption improves baseline colonic microenvironment and impacts the composition and metagenome of the gut microbiota; these effects may prove beneficial in attenuating gut-associated diseases (8,9). In human studies, beans consumed one out of three meals, attenuates postprandial insulin and moderately enhances postprandial antioxidant endpoints in adults with metabolic syndrome (10); furthermore, even a 1/2 cup of beans can produce reductions in postprandial glycemia among healthy adult women (11).
Taken together, these findings strongly suggest that the addition of functional foods at the daily diet diminishes oxidative stress and may be an alternative to prevent complications associated with obesity and diabetes. Therefore, in the present study, the objective was to compare the metabolic and antioxidant effects of the daily consumption of a bean-fiber fortified bar in healthy Mexican subjects.
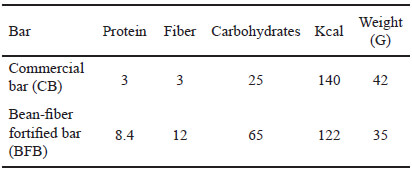
We included a total of 60 healthy subjects, from 18 to 65 years old, who were randomly distributed into two groups. One of them consumed a daily bean-fiber fortified bar (BFB-group) while the other consumed a commercial bar (CB-group) for 30 days. The bean-fiber fortified bar contained the following ingredients: rice flour, honey, bean (Phaseolus vulgaris, 15%), amaranth, raisin, blueberry, corn, maltodextrin; whereas the commercial bar (Oats’n Honey, Nature Valley®) contain the following ingredients: whole grain oats (53%), sugar, canola oil, rice flour, honey, salt, brown sugar syrup, baking soda, soy lecithin and natural flavor. The Table 1 shows nutritional information of the bars.
The study was performed in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the Institutional Ethic Committee from the Universidad of Guanajuato (CIDCSIC-1171303). All patients signed an informed consent form for this investigation.
Determination of nutritional intake and anthropometric characteristics.
All participants attended three citations. At the beginning of the trial basal measurements were performed; these measurements were repeated after 15 and 30 days of consumption of the bars. First, the monthly family income was determined just at the beginning of treatment. Second, anthropometric data such as weight, height for BMI, wrist, waist, hip circumference, mid-arm, biceps and triceps folds were recorded. Third, the calorie, protein, lipid and carbohydrate consumption were estimated using the food frequency survey for Mexican people and by dairy consumption. Thus, dietary intakes were assessed by validated 24 h-dietary records. Participants declared all foods and beverages consumed during a 24 h-period: three main meals (breakfast, lunch, dinner) or any other eating occasion. Portion sizes were estimated using validated photographs. Mean daily energy and nutrient intakes were estimated using a published Mexican food composition table by food and nutrition professionals.
Blood samples were collected at basal, 15 and 30 days of consumption of the bars to determine glucose and lipid profile (PL); besides, a glucose tolerance test (GTT) was applied at basal, 30, 60 and 120 min after bar consumption.
In blood, glucose was determined using the glucose oxidase-peroxidase method (Biosystems, Spain); cholesterol, high-density lipoprotein, low-density lipoprotein (LDL), very-low-density lipoprotein and triglycerides were estimated using enzymatic methods (STANBIO Laboratory, Boerne, Texas, USA).
Lipid peroxidation was estimated measuring malondialdehyde (MDA) content by the Thiobarbituric acid-reactive substances (TBARS) assay, whereas the oxidized proteins were quantified by measuring the carbonyls content. Therefore, ten μL of sera were used to quantify the MDA levels by the TBARS assay, and fifteen μL of sera were used to quantify the carbonyls content as we previously described (12).
The statistical analyses were performed with Statistics for Windows 8 (StatSoft, Inc.). Data were analyzed with ANOVA or repeated-measures ANOVA followed by post hoc tests were used to find the differences between groups, or Student’s t-test was performed. The significance level set at p < .05.
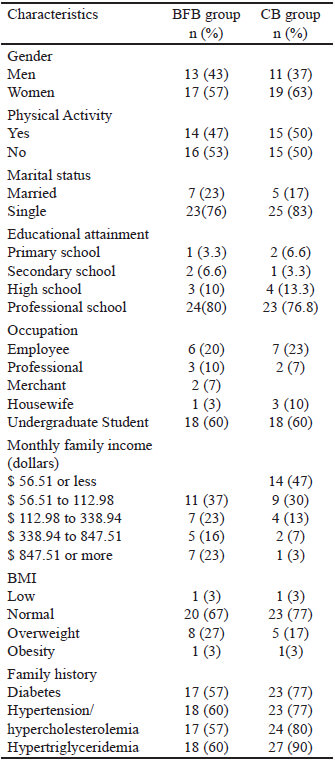
We included 60 healthy subjects aged between 18 and 65; 36 of them were women, and 44 of them were single participants. The monthly family income was less than 56.5 dollars (41.5%), the 51.6% of participants did some kind of physical activity and 65% of participants were undergraduate students (Table 2).
There were no significant differences of anthropometric dimensions between the BFB and CB groups, neither at the basal evaluation nor after the 30-day follow-up assessment (Tables 3 and 4).
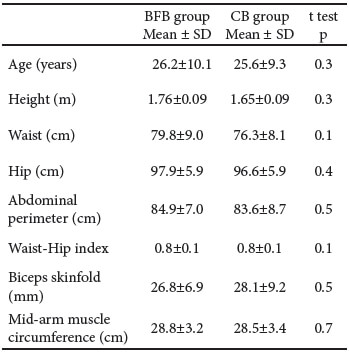
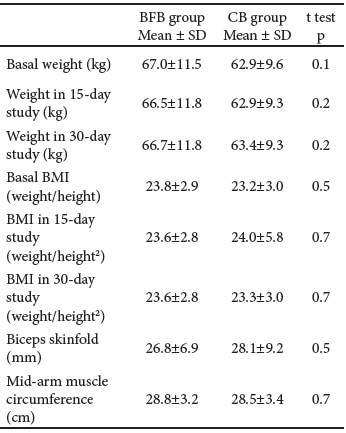
Figure 1 shows the glucose tolerance curves obtained at different times during the study, at the beginning of the trial and at 15 and 30 days after; all of them stayed within normal ranges. In each assessment, the highest concentration of glucose was measured 30 minutes after the administration of the probe, but it downed at 120 minutes to a concentration lower than 100 mg/dL. No significant differences (p>0.05) were found between BFB and CB groups, at initial (A), 15- (B) and 30-day (C) assessments. Time to peak glucose concentration did not differ either between both groups; this positive increment was reduced in both groups and their glucose values were normal.
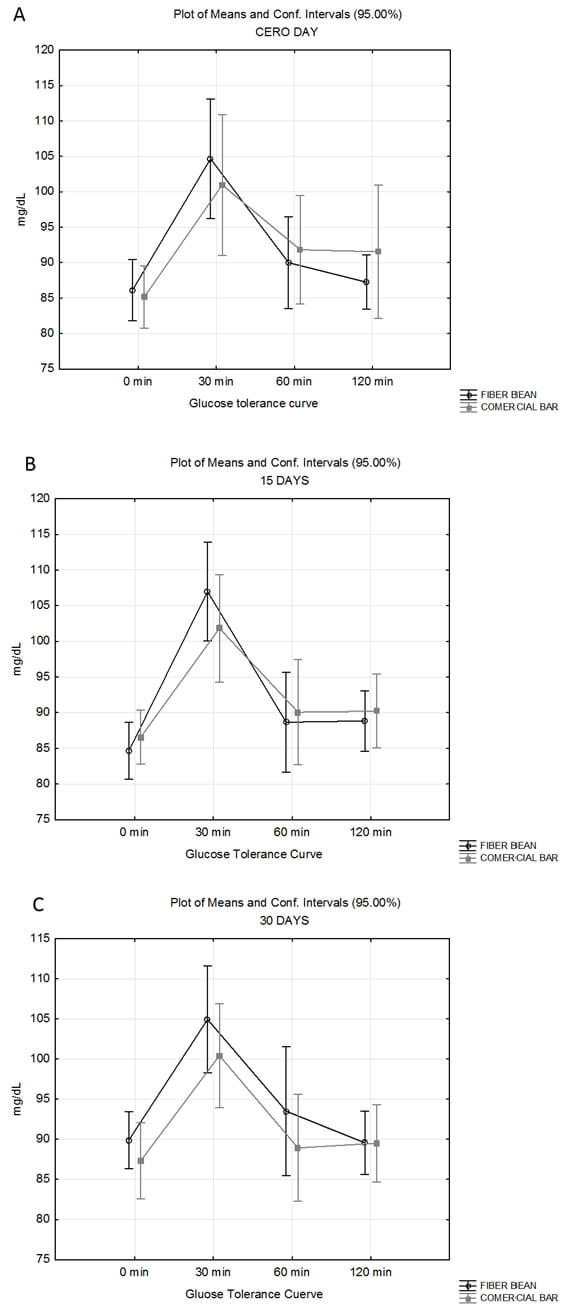
Figure 1 shows the glucose tolerance curves at initial (A), 15-day (B) and 30-day trial times (C). In each measuring period there were not important changes from baseline for glucose concentration at 30, 60, and 120 minutes. No significant difference (p>0.05) was found between BFB and CB bar at initial (A), 15- (B) and 30-day (C) evaluations. Time to peak glucose concentration did not differ between BFB and CB groups in each assessment; these positive increments were slightly reduced in both groups, but the glucose values remained within normal range.
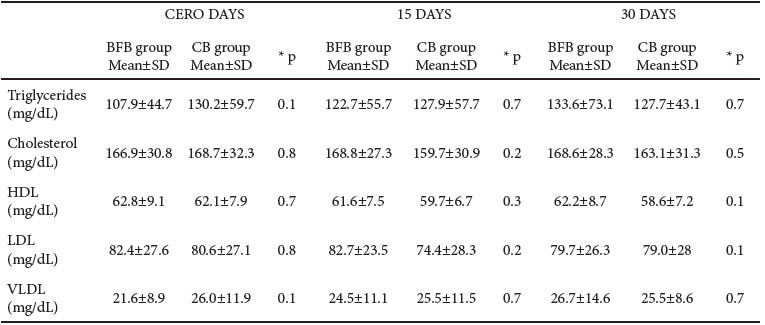
Concerning lipid and cholesterol profiles, there were no significant differences when comparing BFB and CB groups at the 30-day follow-up assessment (Table 5).
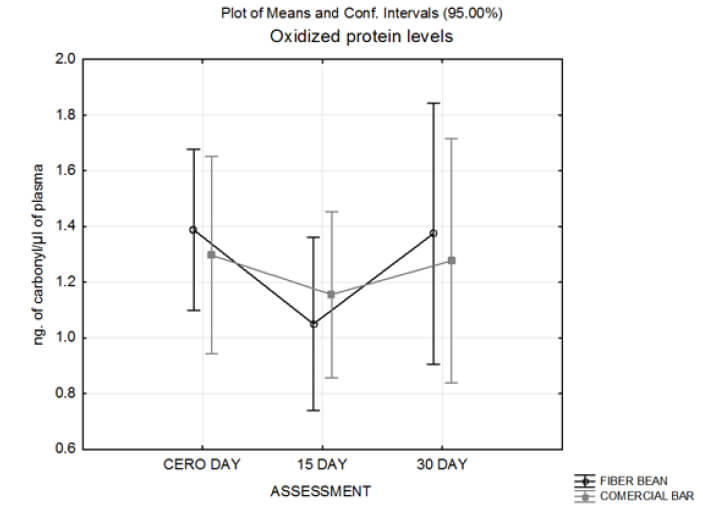
There were no significant differences in the TBARS or carbonyl levels between groups at each of the three assessment periods (basal, 15 and 30-day). Interestingly, the group that consumed the BFB showed higher levels of serum lipid peroxidation at the basal time than CB group, but these levels decreased at the final evaluation (30 days); in contrast, in the CB group these values were increased at the at the end of the trial, annulling the difference between groups (Figure 2).
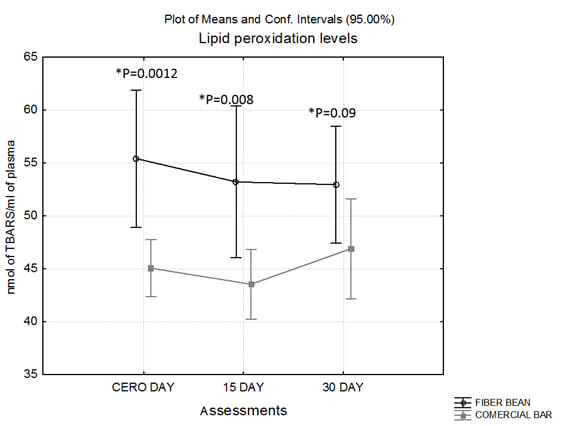
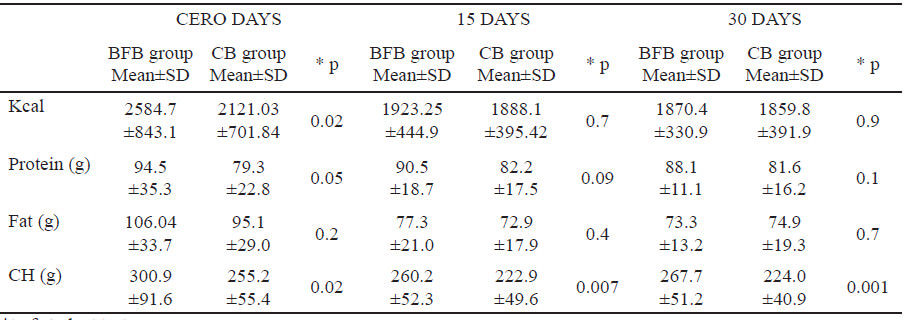
Table 6 shows the average amounts of kilocalories, protein, fat and carbohydrates intake; there was only one difference among the groups in carbohydrate intake at three measurements, BFB group did intake more carbohydrates than CB group, this could explain the higher TBARs levels in BFB group in basal and fifteen-day assessment after having started eating the bar.
Subjects with a weight range of 55-64 kg and 75-86.9 kg have the highest levels of TBARS at basal assessment, but this difference was not remarkable in the two last evaluations (Table 7). It can be assumed that the consumption of the fiber bean bar can attenuate the oxidation attributed to the high consumption of carbohydrates and overweight. On the other hand, at day cero, subjects with a weight range of 65-74 kg in the BFB group have lower of TBARS levels than those in 55-64 Kg and 75-86.9 Kg ranges, but these differences did not appear at the final evaluation (Table 7).
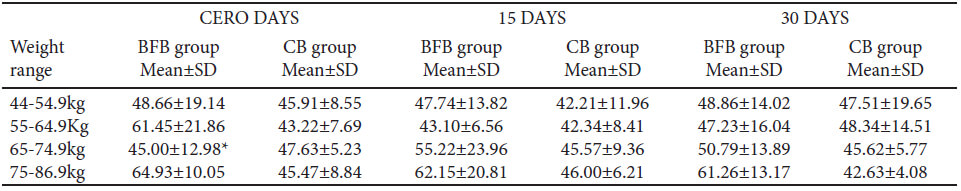
Regarding oxidized proteins in serum, statistical differences were not found between the groups at each assessment, during the bar consumption trial (Figure 3). Carbonyl concentration tend to be higher for the two last assessments in subjects with a weight range of 64-74 Kg in the CB group (1.39 ± 0.89 ng/μL in basal, 1.74 ± 1.11 ng/μL in 15-day, and 2.14 ± 1.85 ng/μL in 30-day assessments).
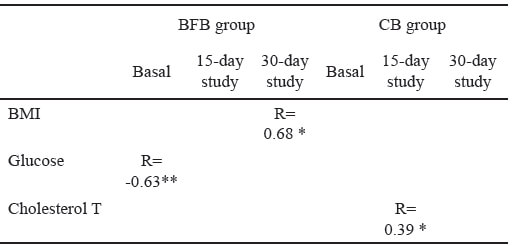
For the BFB group, the basal levels of TBARS were inversely associated with fasting glucose (R=0.63, p<0.001); however, this association did not remain for the next two measurements. At 15 days after starting bar consumption, TBARS levels were directly associated with total cholesterol only in CB group (R=0.39, p<0.05), and 30 days after, with BMI in BFB group (R=0.68, p<0,05) (Table 8).
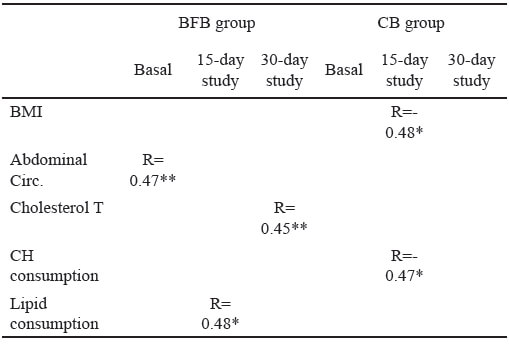
In BFB group, carbonyl levels were associated with abdominal circumference (R=0.47, p<0,001), lipid consumption (R=0.48, p<0.05) and total cholesterol (R=0.45, p<0.001) at basal, 15-day and 30-day assessments, respectively; in CB group, carbonyl levels were inversely associated with BMI (R=0.48, p<0.05), carbohydrates consumption (R=0.47, p<0.05) after 15 days of bar consumption (Table 9).
Several studies affirm that pinto beans can be included in diet, on a daily basis, in a reasonable quantity to provide health benefits that include lowering serum lipoproteins and improving risk factors for coronary heart disease (6, 13). In this study, we did not find reduction in serum lipoprotein after the 30-day follow-up assessment.
BFB showed higher levels of serum lipid peroxidation (TBARS) in basal and fifteen-day samples than CB; perhaps, the higher intake of carbohydrates in the former group promoted it. In the same group, subjects with a weight range of 55-64 kg and 75-86.9 kg have the highest levels of TBARs at basal assessment; this may be associated to a high carbohydrate consumption at basal time; however, levels of TBARS decreased at the final evaluation (30 days after), annulling the difference between both groups and the difference linked with weight range. It can be assumed that, in this study, the consumption of the fiber bean bar can attenuate the oxidation caused by diet and weight; this result agrees with a clinical trials which reported that the dietary supplement of a Phaseolus vulgaris extract to subjects with a carbohydrate-rich, 2000 to 2200-calorie diet, produced; after 30 days of treatment, significant reductions in body weight (4 %), fat mass (10 %), and waist/hip circumferences (3/1.3 %, respectively) compared to the baseline data (14).
Many studies have showed that beans contain substantial amounts of phenolic acids and flavonoids; specially, red, black, and blue-violet coloured beans show also anthocyanins, such as delphinidin and cyaniding; these compounds possess a very strong antioxidant and anti-free-radical activities (15). The antioxidant effect and total phenolic content of common beans had been previously reported, showing that pigmented beans had generally major antioxidant effect and higher amount of total polyphenols, with respect to the nonpigmented varieties (16,17). Other study considers the potential of Phaseolus vulgaris as a natural alternative treatment of some metabolic alterations associated with obesity (18-20). This is the first study in Mexico which evaluate the antioxidant capacity of bean-fiber-bar (Phaseolus vulgaris) consumption.
The basal TBARS levels in this study were inversely associated with fasting glucose in BFB ; however, this association does not remain for the next two measurements. TBARs levels, after 15 days bar consumption, were directly associated with total cholesterol in CB, and after 30 days, with BMI in BFB group. Previous evidence about the relationship between bean consumption and levels of TBARs does not exist; however, some studies show evidence indicating that the inclusion of black beans in a typical Western-style meal attenuates postprandial insulin and moderately enhances postprandial antioxidant endpoints (total Oxygen Radical Absorbance, and Oxidized LDL) in adults with metabolic syndrome, which could only be partly explained by fiber content and antioxidant capacity properties (14). In other study, 30 overweight subjects used Beanblock® for at least 12 weeks; Beanblock® is a standardized dry extract obtained from a selected bean variety of Phaseolus vulgaris Oxidative stress was significantly decreased in this case (21). Regarding oxidized proteins in serum, in this study some statistical differences were found between groups at each one of the three assessments during consumption of the bars; but the carbonyl levels were positively associated with total cholesterol at 30 days after having started consumption of the bean bar. In another previous study, the level of advance oxidation protein products (mAOPPs) has been positively correlated with triglycerides and negatively correlated with high-density lipoprotein cholesterol. But there was no correlation of this marker of protein oxidation with biomarkers of lipid peroxidation (22). In this study, carbonyl levels were directly associated with basal abdominal circumference in subjects in the BFB group and negatively with BMI in subjects belonging to CB group 15-days after bar consumption. Some subjects in BFB group presented central obesity, and this could be an indicator about lifestyle and dietary habits; high-fat, high-carbohydrate meals induce a significantly more prolonged and greater oxidative and inflammatory stress in the obese (23,24); analyzing the average amount of lipids consumed during the first two weeks by subjects belonging to the BFB group, the dietary intake of fat was 33% of total calories. The World Health Organization recommends that lipids must cover between 15 and 30% of the total daily caloric intake and, in Mexico, the National Institute of Medical Science and Nutrition Salvador Zubiran recommended that energy obtained from lipids must be about 25% (25).
In conclusion, consumption for 30 days of fiber bean bars did not alter glucose or PL levels, so their intake is safe; furthermore, lipid peroxidation onset in the BFB group was reduced after 30-day consumption of the fiber bean bars, these levels were inversely related to fasting glucose levels and directly related to BMI and total serum cholesterol levels: These results support the use of these bars like a functional food.
This study was supported by National Institute of Forestry, Agricultural and Livestock Research (INIFAP, Bajío), special grants to Research and Postgrade Support Office of Universidad de Guanajuato (DAIP).
The authors declare no conflict of interest
Recibido: 13/04/2019
Aceptado: 02/09/2019