 ,
Sandra Neli Jiménez-García2
,
Sandra Neli Jiménez-García2  ,
José Enrique Botello-Álvarez1
,
José Enrique Botello-Álvarez1  ,
Rita Miranda-López1
,
Rita Miranda-López1 
Mango is the second most commercialized tropical fruit in the world, and Mexico is the major exporter. In terms of mango production, Manila´s variety represents a quarter of the total mangoes production in Mexico. However, the changes that occur on the phenolic compounds during the Manila mango ripening process are unknown. Quantitative analysis of the major phenolic compounds was conducted at different maturity stages, using several spectrophotometric measurements and by high-performance liquid chromatography (HPLC). At the late ripening stage was observed the biggest content in pulp and peel of total phenols (577 and 10547 mg EAG /100 g), flavonoids (95.33 and 537 mg EQ/100 g), and antioxidant capacity by DPPH (25 and 347 mmol TE/100 g). Some bioactive compounds achieve their highest values at optimal consumption ripening. Although they diminish when the fruit reaches a senescence appearance. This is the first study to prove that mangiferin by itself shows a higher correlation in antioxidant capacity compared to other phenolic compounds in mango peel, and this suggest that phenolic compounds may have an important role in the postharvest antioxidant metabolism in Manila mango fruit. On the other hand, the results show that the peel compared to the pulp contains higher amounts of total phenols, flavonoids, gallic acid, mangiferin and antioxidant capacity, so its use as an ingredient in the preparation of functional food products is recommended. More studies are needed to go in-depth in the changes of the content of phytochemicals during the ripening process in the peel and pulp mango, which ones could be caused by the hormones responsible for ripening in the fruit, such as ethylene, and bioavailability of these compounds at different stages of maturation. Arch Latinoam Nutr 2020; 70(4): 269-281.
Key words: Antioxidant capacity, bioactive compounds, by-products of mango, Mangifera indica L., phytochemicals, and ripening.
El mango es la segunda fruta tropical más comercializada del mundo y México es el principal exportador. En términos de producción de mango, la variedad Manila representa una cuarta parte de la producción total de mangos en México. Sin embargo, se desconocen los cambios que ocurren en los compuestos fenólicos durante el proceso de maduración del mango Manila. El análisis cuantitativo de los principales compuestos fenólicos se realizó en diferentes etapas de madurez, utilizando varias medidas espectrofotométricas y mediante cromatografía líquida de alta resolución (HPLC). En la etapa de madurez tardía se observó el mayor contenido en pulpa y cáscara de fenoles totales (577 y 10547 mg EAG / 100 g), flavonoides (95.33 y 537 mg EQ / 100 g) y capacidad antioxidante por DPPH (25 y 347 mmol TE / 100 g). Algunos compuestos bioactivos alcanzan sus valores más altos en el punto de madurez óptima. Aunque disminuyen cuando el fruto adquiere una apariencia de senescencia. Este es el primer estudio que demuestra que la mangiferina por sí misma presenta una alta correlación con la capacidad antioxidante en comparación con otros compuestos fenólicos de la cáscara de mango, y esto sugiere que los compuestos fenólicos pueden tener un papel importante en el metabolismo antioxidante postcosecha en el mango Manila. Por otro lado, los resultados muestran que la cáscara comparada con la pulpa contiene mayores cantidades de fenoles totales, flavonoides, ácido gálico, mangiferin y capacidad antioxidante por DPPH, por lo que se recomienda su uso como ingrediente en la elaboración de productos alimenticios fucionales. Se necesitan más estudios para profundizar en los cambios del contenido de fitoquímicos durante el proceso de maduración en la cáscara y pulpa del mango, los cuáles podrían ser provocados por las hormonas responsables de la maduración en el fruto, como el etileno, y la biodisponibilidad de estos compuestos en diferentes etapas de maduración. Arch Latinoam Nutr 2020; 70(4): 269-281.
Palabras clave: Capacidad antioxidante, compuestos bioactivos, sub-productos del mango, Mangifera indica L., fitoquímicos y maduración.
https://doi.org/10.37527/2020.70.4.005
Autor para la correspondencia: Rita Miranda-López, email: [email protected].
In Mexico, mango is a fruit with high economic impact since worldwide ranks first as an exporter, with an annual average of 12-15% of its total production. In 2019 the profit from its export increased to 422 million dollars (1).
Mango is consumed either fresh or processed in several food products, which generates large amounts of residual material that cannot be incorporated into food. These residues, also called by-products, compromise around 20% of the mango's weight, represented by seed and peel (pericarp). Therefore, food science has focused on the search for new ingredients derived from fruits and vegetables, emphasizing foods with abundant bioactive compounds that have potential health benefits. Per example, gallic, hydroxybenzoic, hydroxycinnamic and vanillic acids are present in mango; these acids can be easily absorbed in the small intestine and act as potential antioxidant (2). Mangiferin has a positive effect on lipids metabolism and in glycemic control specially in diabetic persons (3).
In this context, several studies have begun to show the health benefits of mango consumption. According to Abbasi (4) several types of mango peel extract grown in China inhibited proliferation of hepatocellular carcinoma in vitro (HepG2 cell line). Rodríguez-González (5) showed that fiber mango pulp (0.5 g per kilogram of weight per day in a DM1 rat model) increased serum insulin secretion levels, and also reduces hepatic steatosis. Moreover, in overweight subjects, mango pulp consumption can reduce oxidative stress and triglyceride levels in serum (6). Considering the previous scientific evidence, it can be said that mango pulp and peel are foods with functional potential, since in addition to its high vitamin content, mango has a significant concentration of bioactive compounds such as total phenols, flavonoids, carotenes, tannins and acids phenolic compounds compared to other "tropical" fruits such as grape, guava and pineapple pulp (7).
On the other hand, in Mexico all commercial mango varieties belong to the Mangifera indica L. species; consequently, there is a wide fruit diversity, among them are Keitt, Kent, Ataulfo, Paraiso, Palmer, Tommy Atkins and Manila (8). This last variety represents 26% of the total production of mango fruit in Mexico (9). The mango is considered a climatic type of fruit. There is very limited information in the scientific literature regarding changes on the content of the polyphenols present in its pulp and peel during the ripening process. Hence the present study aims to provide essential information to favor and encourage agricultural innovation initiatives focused for the use of this fruit, which is of great importance for the development of biotechnological strategies designed to utilize mango’s peel and pulp.
All the reagents utilized, in the different analysis in this study, were of analytical and HPLC grades. They were provided by a commercial brand.
Spectrophotometric measurements were performed on a UV-Vis double beam GENESYS 10S single cell holder spectrometer (Thermo Fisher Scientific TM, USA). An HP 1200 series HPLC system (Hewlett-Packard, Palo Alto, USA) equipped with a diode array detector (DAD) and Discovery HSC C18 column Sigma-Aldrich (St. Luis, MO) reverse-phase column (250 mm x 4.6 mm) with a spherical particle size of 5 μm was used in the High-performance liquid chromatography assay.
The Manila mango was acquired during the months of April (harvest time 1) and May (harvest time 2), from the municipality of Alcozauca, Guerrero, Mexico. Once the fruit arrived at Celaya, Guanajuato, it was selected according to its color and external appearance, considering the mangoes with physiological ripening stage according to the Mexican standard “NMX-FF-058-SCFI-2006” (10) and in conjunction to Palafox-Carlos (11) having a ripening stage of R1 (0-10 % of yellow pigmentation on the surface of the fruit). Those fruits with defects or some visible physiological irregularity were discarded from the study.
Once in the laboratory, the fruits were sanitized with sodium hypochlorite (NaClO) at a concentration of 200 ppm, dried carefully with absorbent paper, and then stored during 30 days at a temperature of 16 °C and relative humidity of 50 %, to resemble the marketing conditions that occur at the “Bajio” region from Mexico. The fruits were sampled in the following days: day zero (D0), five (D5), fifteen (D15), twenty-five (D25), and thirteen (D30). It is worth mentioning that D0 was considered the day of the arrival of the fruit at Celaya, Gto, and therefore at the laboratory (18 h after being harvested).
Pulp and peel were removed by hand from the mango and separately were freeze-dried LabconcoFreeZone® 4.5 model 10404A 7750000 (LABCONCO, Kansas City, MO). Preparation extract was determined according to a modified method described by Ajila (12). Mango pulp and peel freeze-dried samples (0.5 g) were macerated in 10 mL of methanol for 24 h at 40 °C in 1500 rpm using a shaking incubator Shel Lab (Sheldon manufacturing, Oregon, USA), centrifuged at 6000 rpm for 15 min at 25 °C in a Hermle centrifuge model Z200-A (Labortechnik Technologies, Wehingen, Germany), and then filtered through number 1 Whatman paper. The supernatant was collected, and this was used to quantify polyphenols.
The total phenolic content of the mango samples (pulp and peel) was determined using the Folin-Ciocalteu colorimetric method, as described by Singleton (13). The absorbance was measured at 765 nm using a spectrophotometer. The total phenolic content of the extract was calculated from the gallic acid standard curve and was expressed as milligrams equivalent of gallic acid per one hundred dry weight (mg EAG/100 g).
The aluminum chloride method was used for flavonoid determination following the method described by Papoti (14). The total flavonoid content was calculated from the quercetin standard curve and was expressed as milligrams equivalent of quercetin per one hundred dry weight (mg EQ/100 g).
Condensed tannin determination is based on the reaction of condensed tannins with vanillin under acid conditions, using catechin as standard. A 2 mL aliquot of freshly prepared vanillin solution (1 g/100 mL) in sulfuric acid 70% was added to 500 μL of mango extract. The mixture was incubated at 20 °C for 15 min and its absorbance was read at 500 nm; (+)-catechin was used for the reference curve, and the results were expressed as mg catechin/g dry weight (mg EC/g) (15).
The anthocyanin content was measurement by the pH differential method (16). The total monomeric anthocyanin was calculated using the molecular weight of cyanidin-3-glucoside (449.2 g mol-1) and peonidin-3-galactoside (498.9 g mol-1), which are the main anthocyanins found in mango, and whose molecular absorptivity are 25 740 mol L-1 at 520 nm wavelength and 48 400 mol L-1 at 532 nm in acidified ethanol buffer. The content in the sample solution was calculated using the equation below:
Monomeric anthocyanin pigment (mg/L)= (A×MW×DF×1000)/(ε×1)
where A is absorbance in the sample, MW is the molecular weight, DF is the dilution factor, and ε is the molar absorptivity; it is important to mention that the equation presented above assumes a pathlength of 1 cm. The results were expressed as mg anthocyanin/100 g dry weight (mg/100 g).
HPLC analysis was performed according to Palafox-Carlos (17). Samples containing phenols were injected automatically into an HP 1200 series HPLC system with a DAD. Absorption spectra for the main peaks were recorded at 280 nm. The HPLC system was equipped with a Discovery HS C18, which was kept at 30 °C. The mobile phase was composed of water and 1% acetic acid (A) and methanol (B), and the elution gradient was 2–100% (B) in 40 min at a flow rate of 0.8 mL min-1 and 30 °C. The injection volume was 20 μL, and the concentration of each compound of interest was calculated with a calibration curve for the following standards: (+)-catechin, (−)-epigallocatechin, mangiferin, gallic acid, and vanillic acid.
To gauge the antioxidant activity, three methods were used: 1) DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay, where absorbance values were measured on a spectrophotometer at 517 nm, and results were expressed as mmol Trolox equivalent (TE)/100 g of dry sample (18). 2) ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) radical cation (ABTS+*) scavenging activity assay (19), absorbance values were measured on a spectrophotometer at 734 nm, and the results were expressed as mmol Trolox equivalent (TE)/100 g of dry sample. 3) Ferric reducing antioxidant power (FRAP), methodology described by Griffin (20), the FRAP values were measured on a spectrophotometer at 510 nm and the results estimated in mmol Trolox equivalents (TE)/100 g of dry sample.
Data were statistically analyzed following a randomized design. Results were expressed as means ± SD of six replicates for each experiment, considering both harvest time 1 and harvest time 2 per each evaluation day. Data were analyzed using the one-way ANOVA procedure. The Tukey–Kramer multiple comparison tests were used. Standard deviation and variance coefficient between data groups were used to determine significant differences between them at p< 0.05. Multiple linear regression evaluates the influence that predictors (X1, X2, X3, ..., Xn) have on a variable response (Y); in these models, R2 (R-squared) provides an estimate of the strength of the relationship between a model and the response variable, the overall F-test compares multiple coefficients simultaneously of different linear models, and together with p-value indicates the level of significance of the model. In the present study, the independent variables were each polyphenol evaluated and the dependent variable was the antioxidant capacity. It should be noted that, all the statistical analyses in this study were achieved by using the software IBM, SPSS v. 23 for Windows.
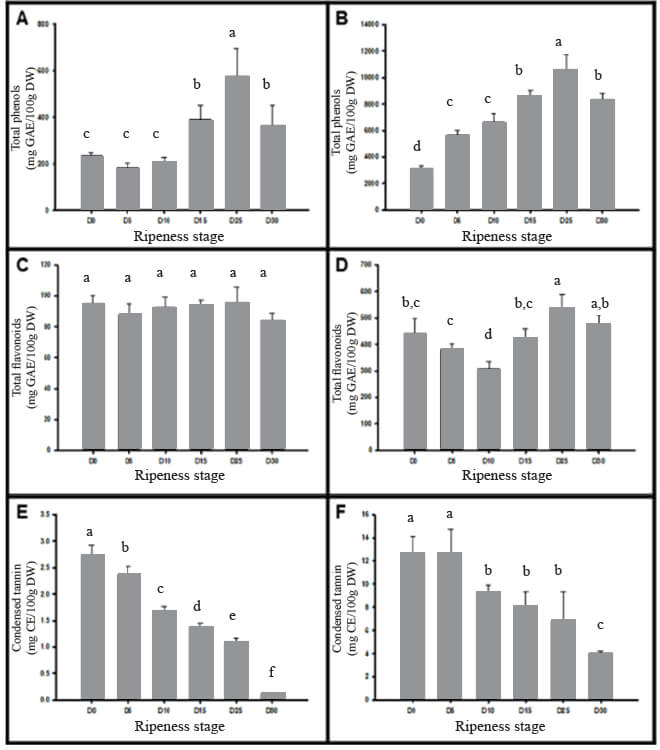
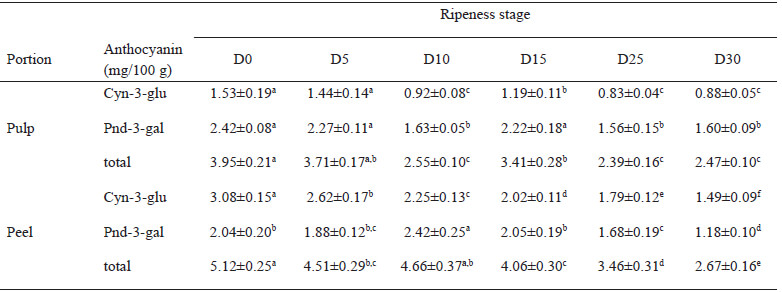
Figure 1 contains the phenolic compounds that were evaluated in Manila mango pulp and peel at different ripening stages, from D0 to D30. In regards to the total phenolic content at day D0 the values were 236.9 and 3154.8 mg de EAG/100g in pulp and peel, respectively; although increases as ripening does (D25), and the total phenolic content in both pulp and peel decreases as senescence is reached (D30) (Figure 1.A and 1.B). The flavonoids values in Manila mango pulp varied from 84.2 to 94.6 mg EQ/100 g, and in the peel from 306.3 to 537.2 mg EQ/100 g (Figure 1.C and 1.D). In all the ripening stages, flavonoids were lower in pulp (4-5 times) than in peel. At the same time, tannin content was also reduced as mango accomplishes higher maturity, the tannin content in pulp and peel were 2.7±0.2 and 12.6±1.5 mg EC/g, respectively (Figure 1.E and 1.F). With regards to the anthocyanin content in pulp and peel of Manila mango (Table 1), this phytochemical decreases during ripening by up to 60 % in pulp and 52 % in the peel. The anthocyanin content was also estimated to be higher in the peel samples than in the pulp.
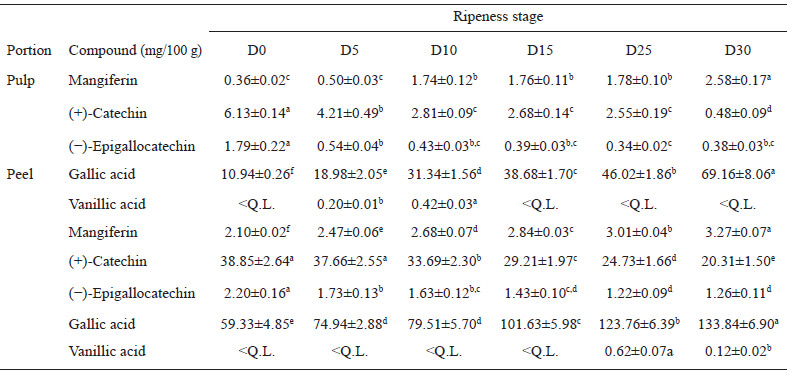
The quantification of phenolic compounds by HPLC is shown in Table 2. Being gallic acid the most abundant polyphenol compound found in the mango samples, followed by catechin. Mangiferin values increases as ripeness does, in both pulp (0.36 – 2.58 mg/100 mg) and peels (2.10 -3.27 mg/100 mg), as well as, the content of catechin and epicatechin gallate in peel was 7-10 times higher compared to the content of its pulp. Although vanillic acid was detected in all the samples, in many of them the concentrations were less than the quantifiable limit established (< L.Q.) in the calibration standard curve.
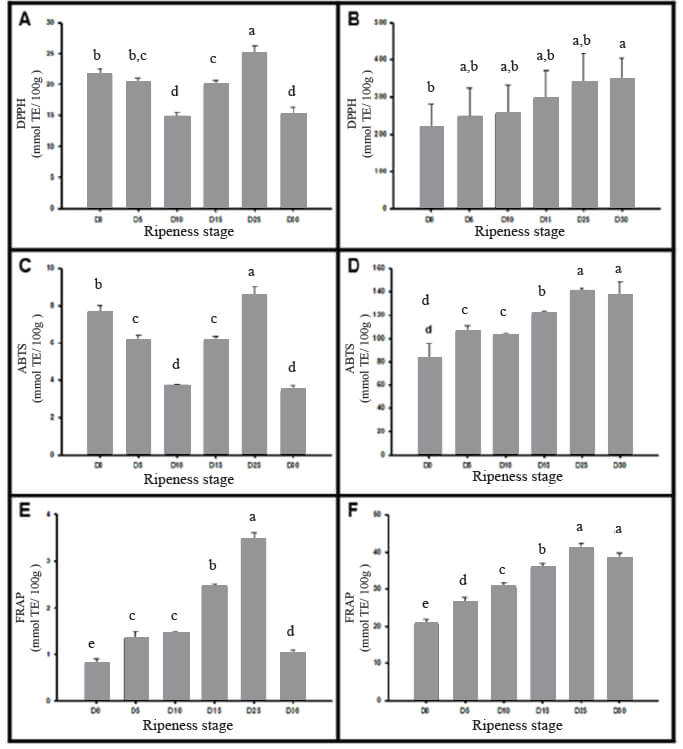
The results of the three antiradical assays are shown in Figure 2, which was observed as the ripening increase the antioxidant capacity increases too. The values in the peel was 4–10 times higher than in the pulp. Figure 2.A shows electron donation of antioxidants to neutralize the DPPH radical in the pulp and peel of Manila mango, whose maximum values were 25.0±1.2 mmol/100 g and 347.4±56.6 mmol/100 g respectively. Figure 2.B shows the low oxidation of ABTS+ by the increase of antioxidant capacity during fruit ripening in mango, peel having values between 82.7±12.7 to 140±2.7 mmol TE per 100 g DW. Finally, at D25 Manila mango holding the highest scavenging activity of Fe3+ in peel with 40.8±1.6 mmol/100 g and 3.5±0.1 mmol/100g in the pulp.
The influence of each polyphenol evaluated was weighed on the antioxidant capacity by using the simple and multiple linear regression model (Table 3). In the case of mango peel, it can be observed that by employing simple linear regression all the polyphenols evaluated participate significantly (p <0.05) by themselves except for vanillin acid, which only showed a significant correlation (p <0.05) for the antioxidant capacity evaluated by the ABTS method. The single phenolic acids are the ones with the greatest contribution.
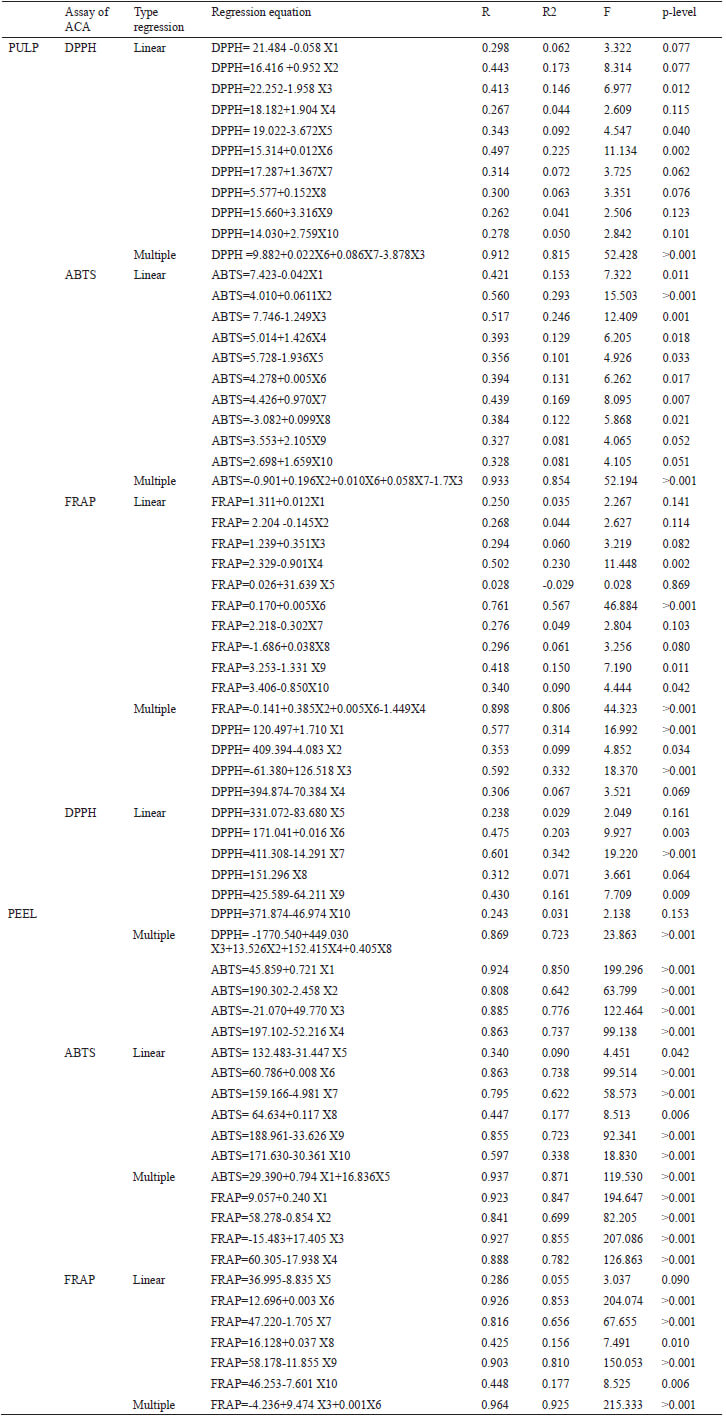
Moreover, mangiferin contributes to a majority way on the antioxidant capacity evaluated by the DPPH and FRAP method, with standardized Beta coefficients of 2.1% and 0.5%, respectively (data no show), and in the pulp of this variety, mangiferin was profiled within multiple regression models to explain the antioxidant capacity evaluated by the different methods. Furthermore, the interaction between catechin, epicatechin, and total phenols explains in 80% the value of the antioxidant capacity in pulp by the FRAP method.
The content of total phenolic in the present study are according to Maldonado-Astudillo (21), who reported similar results (544 to 416 mg EAG/100g) in the pulp of this variety grown in another state of Mexico. The changes in total phenolic is triggered by the loss of water during the ripening of the climatic fruits and it is denoted by its softness increment, perhaps it can be explained by the progressive structural rearrangement of the cell wall, loss of pectin and hemicelluloses interconnection, which is important because some phenolic compounds are trapped in the pectin chains of the cell wall (22).
The values of flavonoids in the peel are consistent with Foluso (23), who indicate that the total flavonoid content in mango peel is 449 mg EQ/100 g. In contrast, the pulp of several citrus flavonoid productions decreases or even stops during cell elongation and subsequent of leaves and ripening (11), which could explain why in Manila mango the content remains constant during the ripening of the pulp; additionally, flavonoids are great antioxidants with health benefit effects; there are known to hold antiproliferative and antitumor growth properties, making them a potential anticancer agent (24).
The values of tannin or proanthocyanidin in Manila mango are similar to those stated by Rashmi (25), who reported content of 3.5-10.6 mg/g and 8-14 mg/g in pulp and peel, respectively in seven Indian mango varieties (Banganapaglli, Alphonso, Totapuri, Mylupilian, Dusheri, Langra, and EC-95862). Unripe fruits usually have high tannin content that helps to protect them from environmental factors and to prevent them from herbivores animals. It appears that the degree of polymerization changes during fruit ripening; however, the content varies based on the type of fruit and the environmental factors, like temperature and humidity, among others (26).
The anthocyanin content in Manila mango is in agreement with López-Cobo (27), who reported a concentration range of 1.1 to 5.3 mg/100 g in mango peel, and in pulp is approximately about half to ten times less compared to its peel. The peonidin content has a higher concentration than cyanidin because the cyanidin contain the o-diphenol structure on the B ring, which is more sensitive to oxidation than other flavonoids. Anthocyanins have also been seeming to protect flavonoids from oxidation, which play a greater role in plants to attract insects in order to spread pollen and seeds, encouraging the survival and reproduction of the plants (28).
Regarding to HPLC quantification, this study concur with other studies finding gallic acid as the major polyphenol present in mango varieties such as the “Keiit” (47 mg/100 g) and “Kensington pride” (906 mg/100 g) in peel (29). The importance of gallic acid in human health is that it can exert its cytotoxic and antitumor effect because of modulation in antioxidant/pro-oxidant balance (30).
Mangiferin content is agree with 11 varieties of mango reported from China, it was found between 0.2-20 mg/100 g in pulp and 4-749 mg/100 g in the peel (31). Some authors suggest that mangiferin increases as fruit mature, perhaps due to the xanthophylls generation, as products of the condensation of flavanols with glyoxylic acid, lactone, and xanthylium structures during ripening (24). Studies have suggested that mangiferin ameliorates insulin resistance by the inhibition of both glucose uptake and insulin signaling, which facilitates the translocation of glucose transporter type 4 and stimulates glucose uptake (32).
The content of catechin in peel are similar to the ones reported by Da Silva Sauthier (33) which indicates a value of 22.4 mg/100 g in peel and 7.71 mg/100 g in the pulp of "Rosa" and "Espada" mangos, respectively. The decrement of catechin and epicatechin during ripening, could be because the corresponding quinone methide is catalyzed by the enzyme PPO (polyphenol oxidase), which is known to increase significantly as the mango fruit matures (26). Regarding the health benefits associated with these compounds, some authors have pointed its cardioprotective effect, such as reducing levels of cholesterol and very-low-density lipoprotein (VLDL) (34).
In addition, the particular case of vanillic acid presents a similar pattern with other mango varieties, this phenomenon has been reported by Da Silva Sauthier (33). In other studies, the concentration of this compound is 1.59 mg/100 g, 0.59 mg/100 g, and 2.67 mg/100 g for Keiit, Osteen, and Sensación variety, respectively (27). Variations regarding the concentrations of the quantified compounds can be attributed to ontogenetic factors, that can modify the production of secondary metabolites by directly influencing the expression of the fruit genes (35).
The fact of a higher antioxidant capacity in the peel of mango which agrees with the hypothesis of the peel of mango has a role in defense (delay, control or inhibit) against various biotic and abiotic stress conditions during fruit development. It can be seen that the antioxidant potential by DPPH assay in the pulp of the Manila mango is within the ranges of 20-75 mmol/100 g reported in six different flesh of Thailand mango cultivars (36). In contrast, the antioxidant potential in the peel is lower compared to Blancas-Benitez (2), who report concentrations of 790 mmol/100 g. These differences may be due to the maturity stage of the plant tissue or exposure to biotic and abiotic stresses can directly influence the genes that express the metabolic enzymes which are required for the synthesis of several compounds that directly influence the antioxidant potential.
The antioxidant capacity measurement by ABTS assay were comparable to the ones described by Blancas-Benitez (2), with concentrations of 116.01 mmol/100 g in the peel of Mexican mango Ataulfo variety, and pulp presented ranges between 3.4±0.3 to 8.5±0.5 mmol/100 g DW. The increase in antioxidant power may be exposed to high storage since this process exhibit elevated levels of oxidation in itself; before disrupting cellular membranes and release naturally sequestered oxidants during fruit respiration process. In parallel, the result of FRAP assay in the present study is smaller than that reported in Australian mangos peel (concentrations of 2-25.5 mg/g of ascorbic acid equivalents), which can be explained by the type of standard used to quantify it.
Lastly, the greater correlation between vanillic acid and antioxidant capacity in linear regression may be due to the fact that the major polyphenols in Manila mango are phenolic acids, which are small molecules, and therefore can react with the radical and be more reactive, resulting in higher antioxidant capacity by FRAP assay. Other reason for the FRAP assay may be promoted principally by flavonoid content in multiple regression is that only quercetin and myricetin found to chelate Fe2+, even though others flavonoids like kaempferol, quercetin, myricetin, luteolin, naringenin, and catechin are capable of complexing Fe3+ (37).
The results indicate that the mechanism of action by phenols in pulp and peel of Manila mango is variable and, including electron-donating ability, hydrogen atom transfer, and the chelation of catalytic metals; it´s important to consider that the effect of antioxidant concentration depends on many intrinsic factors, including the structure of the antioxidant, and oxidation conditions during mango ripening process. The mechanism by which antioxidants exert their effects may vary depending on the polyphenolic compositional of the food, including its minor components in response to the initiation of ripening as described in Figure 3.
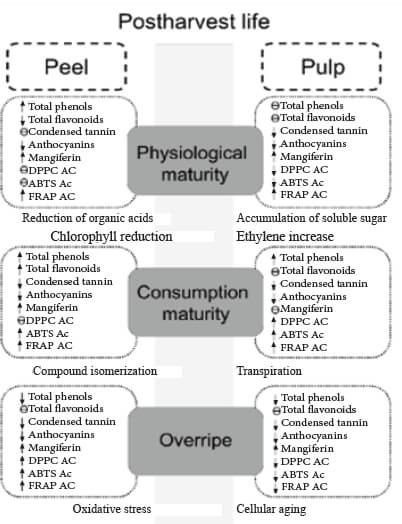
Furthermore, the health benefit effects of eating plant foods have been ascribed, in part, to the presence of phenolics, which are associated with other mechanisms of action in addition to its antioxidant capacity, such as apoptosis of cancerous cells, effects on cell differentiation, blocking the formation of N- nitrosamine, and affecting enzyme activity, among others (35); thus, the action mechanism by which beneficial health effects of phenolics are rendered may follow one or more mechanisms.
The first profile of phytochemical compounds in mango Manila during several ripeness stages was obtained. Likewise, this study confirm mango peel is considered a major location of polyphenols within the whole fruit, which gives the possibility of the mango peel will be able to integrate in the food industry. Also, Manila mango peel and pulp has a high bioactive potential due to its mangiferin content and to the antioxidant expression of the other functional compounds like flavonoids and tannins, in as much as the aforementioned phytochemicals showed a significant correlation with antioxidant capacity.
It should be noted, that the knowledge of the concentration of bioactive structures in the peel of Manila mango allows us to obtain information to implement extraction and drying techniques to give it greater added value as a functional food and to compete in the international market with high-quality products.
The authors declare that they have not conflicts of interest with the contents of this paper.
The authors thank CONACyT (Consejo Nacional de Ciencia y Tecnologia) for the graduate scholarship number 291236, and also to the National Technological from Mexico (Tecnologico Nacional de Mexico) for its support to this research (grant 7668.20-P).
Recibido: 05/10/2020
Aceptado: 10/03/2021