 ,
Laércio Marques da Luz Neto1
,
Laércio Marques da Luz Neto1  ,
Luciana Maria Silva de Seixas Maia2
,
Luciana Maria Silva de Seixas Maia2  ,
Angela Amancio-dos-Santos 3
,
Angela Amancio-dos-Santos 3 
Reflex-ontogeny and intestinal morphometrics were evaluated in Wistar rats whose mothers were fed on a high-fat diet during the perinatal period. Male pups (n=52) formed four experimental groups: NN (pups from mothers with lab chow diet during gestation and lactation); NH (pups from mothers with lab chow diet during pregnancy and high-fat in lactation); HH (pups from mothers with high-fat diet during gestation and lactation); HN (pups from mothers with high-fat diet during pregnancy and lab chow in lactation). The reflex ontogeny, the maturation of physical characteristics and parameters of somatic growth were evaluated during lactation. In addition, the body mass index (BMI), the specific rate of weight gain (SRWG), the Lee index, the weight of the brain and intestinal parameters were analyzed after weaning. High-fat diet during pregnancy (HH and HN groups) delayed the maturation of reflexes and physical characteristics. The high-fat diet affected somatic growth differently, reducing somatic growth parameters in the groups NH and HH and increasing in the HN group. The highest SRWG was found in group HN. SRWG and BMI were reduced in the groups NH and HH. The relative intestinal weight was reduced in the groups NH, HH and HN. The relative length of small intestine was longer in group HN than in group NN. The total height of the mucosa and size of the villous were lower in group HH than in group NN. In conclusion, high-fat diet promoted negative consequences for the development of the nervous and enteric systems of the offspring. Arch Latinoam Nutr 2021; 71(2): 138-148.
Palabras clave: High-fat diet, reflexes ontogeny, somatic growth, rats, intestinal morphometry.
Ontogenia refleja y morfometría intestinal fueron evaluados en crías de ratas Wistar que fueron alimentadas con una dieta alta en grasas durante el período perinatal. Los descendientes machos (n = 52) formaron cuatro grupos experimentales: NN (hijos de madres que utilizaron alimentos de laboratorio durante la gestación y la lactancia); NH (hijos de madres que comieron dieta de laboratorio durante el embarazo y dieta con un alto contenido de grasas en la lactancia); HH (hijos de madres con una dieta alta en grasas durante el embarazo y la lactancia); HN (hijos de madres que comieron una dieta alta en grasas durante el embarazo y comida de laboratorio durante la lactancia). La ontogenia refleja, la maduración de las características físicas y los parámetros de crecimiento somático durante la lactancia fueron evaluados. Además, el índice de masa corporal (IMC), la tasa específica de aumento de peso (SRWG), el índice de Lee, el peso cerebral y los parámetros intestinales fueron analizados después del destete. La dieta alta en grasas durante el embarazo (grupos HH y HN) retrasó la maduración de reflejos y características físicas. La dieta alta en grasas afectó el crecimiento somático de manera diferente, reduciendo los parámetros de crecimiento somático en los grupos NH y HH y aumentando en el grupo HN. El SRWG más grande se encontró en el grupo HN. El SRWG y el IMC se redujeron en los grupos NH y HH. El peso relativo intestinal se redujo en los grupos NH, HH y HN. La longitud relativa del intestino delgado fue mayor en el grupo HN que en el grupo NN. La altura total de la mucosa y el tamaño de las vellosidades fueron menores en el grupo HH que en el grupo NN. En conclusión, la dieta alta en grasas tuvo consecuencias negativas para el desarrollo de los sistemas nervioso y entérico de la prole. Arch Latinoam Nutr 2021; 71(2): 138-148.
Key words: Dieta alta en grasas, ontogenia refleja, crecimiento somático, rata, morfometría intestinal.
https://doi.org/10.37527/2021.71.2.006
Autor para la correspondencia: Angela Amancio-dos-Santos. E-mail: [email protected]
Diets that are rich in sugar and fat are considered more palatable. However, they are harmful for health and associated with obesity and chronic diseases (1). Increasingly, studies have focused on the effects of these diets on adulthood. It is possible that the intake of a high-fat diet by mothers during pregnancy and lactation may be prejudicial to adequate body functioning of the offspring for the whole lifespan (2). Cafeteria diets have been employed to study obesity in rats. These diets consist of highly energetic and highly palatable human foods along with chow diet to trigger diet-induced obesity in laboratory animals (3). The use of cafeteria diet mimics dietary patterns observed in humans (4).
It is well established that malnutrition early in life impairs nervous system development. This system has a development window that is not very flexible. Thus, if the nervous system development occurs in an adverse environment, this system will present sequels that will not be ameliorated even if environment is recovered later (5). This phase is the so-called brain growth spurt and encompasses pre- and early postnatal time-points. In rats, this period occurs in both pregnancy and lactation (three weeks each). Nervous system development can be evaluated through reflex ontogeny (6). By analyzing the consolidation of reflex response, it is possible to identify the sequential steps of normal brain development.
Besides the nervous system, the digestive system can also be affected by malnutrition early in life (7). In rats, many developmental changes in the intestines occur during the third week of life, and there is strong evidence that intrinsic and extrinsic factors, like the quality of diet, could be responsible for a variety of these changes (8). McMenamin et al. (7) found that diet-induced obesity induces peripheral inflammation accompanied by a loss of myenteric neurons. Few studies, however, have investigated the effects of a high-fat diet on the morphological and structural development of the intestine.
Considering all the aforementioned issues, we decided to address the following issues: 1) how does maternal intake of the cafeteria diet during pregnancy and/or lactation alter the reflex ontogeny in the offspring? 2) In which period, pregnancy or lactation, is brain development more susceptible to effects from intake of the cafeteria diet? 3) How does cafeteria diet influence intestine morphometry of offspring?
We found the cafeteria diet delayed reflex ontogeny; high-fat diet during lactation seems to be more harmful to offspring’s development; and, the unbalanced diet impaired intestine morphometric parameters. Thus, it demonstrates the importance of developing public policies to improve the nutrition of populations, especially females in the reproductive age (9). This study reinforces the negative consequences of consuming foods rich in sugar and fat during pregnancy and lactation for offspring, which could result in serious damage to quality of life and longevity.
Experiments were carried out in accordance with the “Principles of Laboratory Animal Care” (National Institutes of Health, USA) and the norms of the Ethics Committee for Animal Research of the University (Protocol number 23076.039142/2012-21). Experiments were done mainly in 2014, but some analysis occurred posteriorly. Wistar rats were housed in polyethylene cages (51 cm×35.5 cm×18.5 cm), under a 12-h light/dark cycle (lights on at 6:00 a.m.) at 22±1 °C with ad libitum access to food and water.
Pregnant females were kept in individual cages and were subjected to one of the following feeding regimes: 1) Lab chow diet (Presence®, Agribrands Purina do Brasil Ltda); or, 2) High-fat diet, which was provided to induce obesity in the animals.
The high-fat diet is a type of cafeteria diet and consists of a normal protein and high fat mixture adapted from Estadella et al. (10). Briefly, it was made up of Lab chow diet (Presence®), plus roasted peanuts, milk chocolate and cornstarch biscuit, in the ratio 3:2:2:1, respectively. Lab chow diet supplied 3.8 kcal/100g (23% proteins, 4% of lipids, and 63% of carbohydrates) while the supplied high-fat diet provided 4.8 kcal/100g (17.93% of proteins, 24.5% lipids and 47.18% carbohydrates).
Male pups born from the dams described above formed the experimental groups as described below:
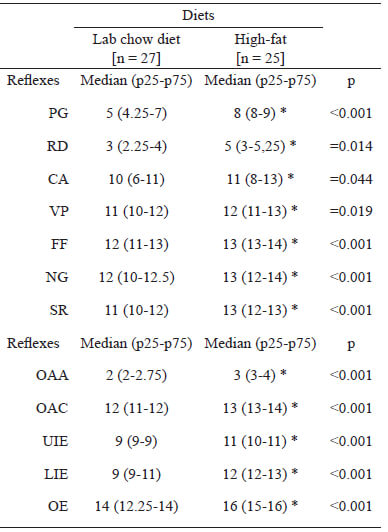
Daily maturation of each animal was analyzed according to Fox’s protocol (6). The following tests were carried out: palm grasp (PG), recovery to decubitus (RD), vibrissa placing (VP), cliff aversion (CA), negative geotaxis (NG), startle response (SR) and the free fall (FF).
The reflexes were investigated from the 1st day of animal's life up to the day of its consolidation; always being observed between 12:00 a.m. and 2:00 p.m. The reflex is considered consolidated when the total response is observed unequivocally. The day of reflex maturation was established as the first day following three consecutive days of complete appearance of the expected reflex response. Evaluations were performed using tools that existed or had been previously developed in the laboratory.
The pups were examined daily (from postnatal 1) to determine the day when complete maturation of the following physical characteristics was displayed: opening of the auditory auricle (OPA); opening of the auditory canal (OAC); eruption of upper and lower incisor teeth (respectively, UIE, and LIE) and opening of the eye (OE). These characteristics were considered as indicators of somatic development.
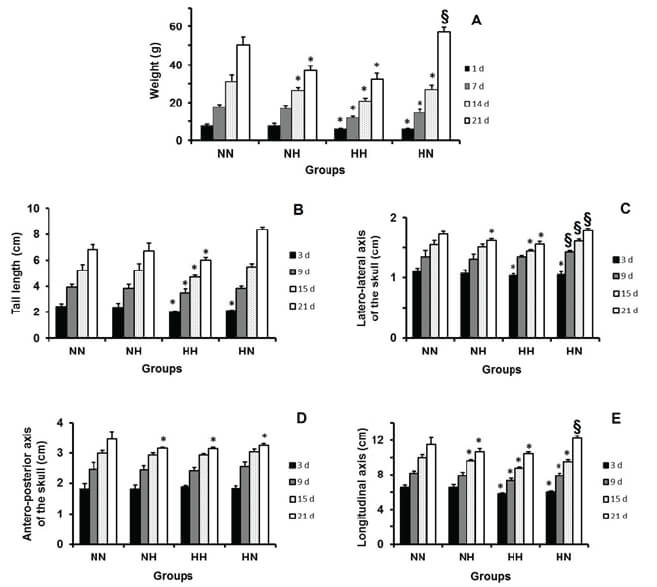
Somatic growth was measured according to Silva et al. (11). The animals were assessed weekly on days 3, 9, 15 and 21, using a digital pachymeter, with an accuracy of 0.01 mm. The following were evaluated: Tail Length (TL); lateral-lateral axis of the skull (LLAS); antero-posterior axis of the skull (APAS); longitudinal axis (LA).
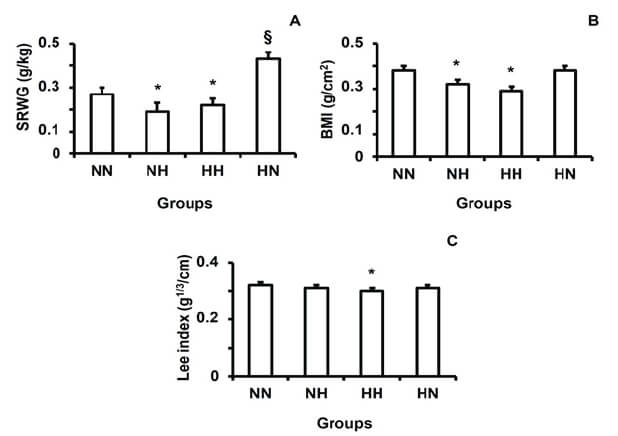
Specific rate of body weight gain (g/Kg) = (dM/M) dt, where
dM = body weight gain during dt
(dt = t2-t1; t = time)
M = weight of the rat in t1
BMI = weight (g)/ [nose-to-anus length (cm)]2
Lee index = 3√weight (g) ∕ nose-to-anus length (cm)
Animals were anaesthetized and sacrificed by overdose of a mixture of the anesthetics urethane (1000 mg/kg) and alpha-chloralose (40 mg/kg). Abdominal organs were removed in a single block and the intestine was separated from the mesentery, and then weighed on a digital scale (sensitivity 0.1 g). Jejunum fragments were washed with 0.9 % saline solution and fixed in 10% buffered formalin, for 48 hours. Transverse sections of intestine were obtained with approximately 5 μm thickness through microtome (Leica). Sections were stained with Hematoxylin-Eosin (HE) and assembled with synthetic resin (Entellan-Merck).
From each histological section, 10 villi without damage were randomly selected. All villi had to be well oriented, with apparent and continuous basal, medial and apical portions. After the capture, using 10X objective, the following parameters were measured:
For these analyses, a microcomputer was used with the analytical image program ImageLab2000, and with the optical microscope Motic BA 200 (10X objective). The program "Image J" was used for the measurement. Additionally, the length of the small intestine was also evaluated, which was measured from the pylorus to the ileocecal junction by using a ruler.
After the sacrifice, the brain was removed and weighed on a digital scale with sensitivity up to 0.1 g. The obtained value was used for relative weight calculation. Then, it was placed in an oven for 72 hours to obtain the dry weight.
The normality measurements were evaluated using the Kolmogorov-Smirnov test. The parametric data were analyzed by analysis of variance (ANOVA) followed by the Tukey test for multiple comparisons. Non-parametric data were analyzed by the Mann-Whitney test for two independent samples. The significance value was considered p < 0.05.
Maternal food consumption was assessed during the experimental period. The mean ± s.d. of female rats’ daily food intake, fed the lab chow diet, was 21.84 g ± 0.74 g during pregnancy and 45.88 g ± 1.81 g during lactation. For the high-fat female rats, the mean ± s.d. consumption was 14.26 g ± 2.22 g and 27.18 g ± 0.94 g during pregnancy and lactation, respectively (Table 1).
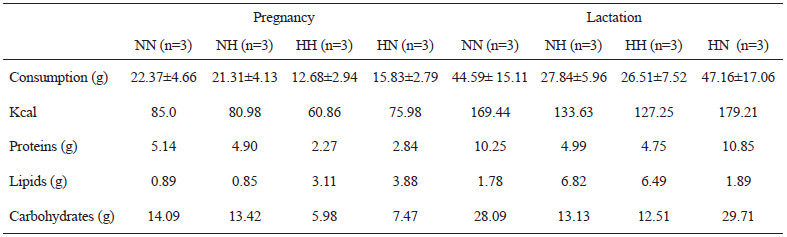
Pups whose dams received high-fat diet during pregnancy (HH and HN) delayed (p<0.05) the maturation of all reflex response, when compared to the control group (NN). Results are shown in Table 2.
Concerning to the tail’s length, there was no significant difference between the animals of the group NH and the control group. The group HH exhibited shorter tail length than the control group during the entire lactation. The HN group presented, initially, an inferior length; but, at the end of lactation, it was superior to the control group (Fig. 1.B).
Regarding the LLAS, the group NH presented a lower value than the control only at the end of lactation (day of weaning). The groups HH and HN presented LLAS lower than the control group at the beginning of lactation. At the end of that period, this reduction remained only in group HH. Interestingly, the group HN started presenting value higher than the control group from the 9th day of lactation (Fig. 1.C).
Regarding the APAS, there was no difference among the groups at the beginning of lactation. However, at the weaning, all experimental groups presented APAS lower than the control group (Fig. 1.D).
Concerning the LA, the group NH measured shorter than the control group from the 14th day of lactation. In the group HH, the values were less than those of the control group during the entire lactation. Regarding the group HN, the LA was reduced up to the 15th day of lactation; however, at weaning, it was larger than that of animals in the control group (Fig.1.E).
The group NH had body weight similar to the control group at the beginning of lactation. However, from the 14th day, this weight became inferior. During the entire lactation, the pups of group HH showed lower weight than those in the control group. The animals of the HN group presented lower body weight than the control group up to the 14th day of lactation. However, at the weaning, the body weight of these animals was higher (Fig. 1.A).
Regarding the specific rate of body weight gain and BMI, both NH and HH were slower than in the control by the end of lactation. In the group HN, the specific rate was faster than in the control group at weaning and BMI did not differ. In relation to the Lee index, only the HH group had reduced values in comparison to the control group (Fig. 2).
Absolute values of wet brain weight did not differ among the groups. However, the relative values showed that the brain weight in groups NH and HH was higher than the control. Dry brain weight was reduced only in the HH group compared to the control group (Table 3).
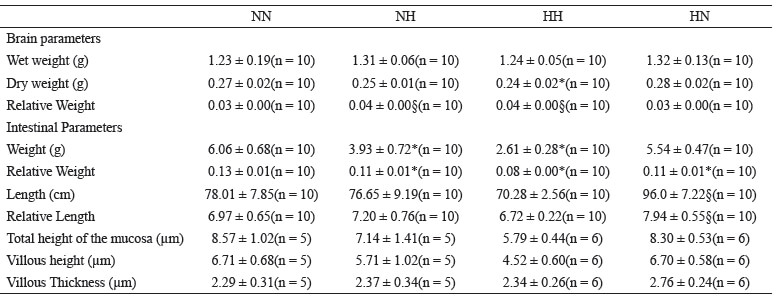
In relation to the absolute weight of the intestine, the groups NH and HH showed lower values than the control group. When the relative weight of the intestine was evaluated, the three experimental groups presented lower weight than the control group. Concerning the length of small intestine, only the group HN presented a mean longer than the control group with respect to both absolute and relative values (Table 3).
Regarding the morphometric parameters of the intestine, the total height of the mucosa and the villous size were reduced only in the HH group compared to the group NN. The thickness of the villi was the same among the groups (Table 3).
Pregnancy and lactation are phases of life when the nervous system of human beings in development is highly vulnerable to environmental insults. Nutritional injuries occurring in these periods can alter the brain structure and function, as evidenced through inadequate reflex ontogeny (13).
In the present study, both the maturation of physical characteristics and the consolidation of reflex response were found to be delayed in those groups whose mothers were fed a high-fat diet during pregnancy. Similar results were observed in the study of Giriko et al. (14). In that study, all indicators of physical maturation were established later in the offspring of dams fed a high-fat diet. Moreover, the consolidation of the reflexes of vibrissa placing, startle response, straightening in free fall and negative geotaxis were also delayed.
It is worth noting that lipids are fundamental for adequate brain development. Thus, it could be assumed that exposure to a high-fat diet early in life could favor nervous system maturation. However, the type of lipids in the diet matters. González & Visentin (15) showed that the type of fatty acid present in the diet interferes with the nervous system development. Animals fed on a diet whose lipid base was hydrogenated vegetable fat, rich in saturated fatty acids, showed delay for opening of the ear canal and of the negative geotaxis reflex, when compared with the control group animals, whose dietary lipid source was soya oil, rich in polyunsaturated fatty acids. Thus, the adequate intake of polyunsaturated fats during childhood is essential for optimal growth and the proper development of the organic functions.
The high-fat diet used in our study was a cafeteria diet made with standard lab chow for rodents, to which the following ingredients had been added: cornstarch biscuit, roasted peanuts and milk chocolate (10). This mixture provided a diet high in fat but deficient in essential fatty acids. Thus, it is possible to test whether the delay in the maturation of the physical characteristics and in the consolidation of reflex responses was due to the inadequacy of this cafeteria diet. The results raised a warning about the quality of food that expectant mothers consume during pregnancy and lactation since this was shown to significantly affect the brain development of the offspring.
Additionally, in this study we found that all the females fed on a high-fat diet presented a reduction in food consumption (Table 1), potentially reducing protein intake. In fact, the protein intake of the rats receiving high-fat diet was 51% and 46.2% of the control rats during pregnancy and lactation, respectively. It has been reported that a low protein diet during pregnancy and lactation induces permanent changes in the structure and functionality of the central nervous system, such as the delay in reflex ontogeny (16). Thus, we can assume that the delay in brain maturation seen in our study was due: a) firstly to the lipid quality of the high-fat diet; b) secondly to the protein deficiency induced by intake reduction while on the diet.
Regarding to the parameters of somatic growth (TL, LLAS, APAS, LA and body weight), it was seen that the maternal intake of high-fat diet during lactation (groups NH and HH), related to length of weaning (21 days of life), resulted in a reduction of almost all the aspects analyzed (except the TL in group NH). It is interesting that the group HN initiated the lactation with low values, as said above. However, all parameters, except APAS, were higher than in the group NN at weaning.
In contrast, in other investigations in which dams were fed on a high-fat diet during pregnancy and/or lactation, pups had increased body weight and growth (17); or, significant difference in these parameters were not found (18). Given the above, it can be seen that the effects of high-fat diet on the somatic growth are not still well established, especially when this diet is offered during the critical period of development. Further studies are necessary to precisely clear the relationship between a high-fat diet and body weight and growth development.
The measures of the cranial axis, LLAS and APAS, are related to the growth and development of the skull and indirectly to the development of the central nervous system (18). LLAS is responsible for the growth and development of the braincase (region of neurocranium), while APAS is directly related to the functions of the eruption of teeth and chewing, being named the viscerocranium region (19). Miller & German (19) found greater involvement of the viscerocranium in relation to the neurocranium in rats subjected to protein malnutrition. Our results agree with these authors, since APAS was reduced in all experimental groups, while LLAS was low in the animals whose mothers were fed on a high-fat diet during lactation (groups NH and HH). This result also reinforces our hypothesis that there may have occurred a secondary protein deficiency from the reduction of the intake of a high-fat diet.
It is interesting to note that group HN gained more body weight than the NN group. These pups may have undergone the phenomenon known as catch-up growth, which can be described as a period of rapid linear growth that follows a period of growth inhibition, leading towards their original growth pattern (20). Further investigations are necessary to clarify this point.
The NH and HH groups presented reductions in SRWG and BMI, while the HN group had the highest SRWG. These results were expected since both those parameters depend on body weight. Concerning the Lee index, a direct relationship between a high-fat diet and this parameter has been reported (21), related to rates of adiposity. In contrast, our findings showed the group HH, that received the high-fat diet throughout the experimental period, had the lowest Lee index. Methodological differences between the studies, however, may have influenced the opposite outcomes. Mali et al. (21) measured the Lee index in animals at 40-90 days old, while our rats were 21 days old.
Regarding brain weight, we found the NH and HH groups presented higher relative brain weight and lower relative intestine weight than the group NN. This result indicates that the brain was less affected by the high-fat maternal intake compared to the body. It also demonstrates that the impact of a high-fat diet on the brain is strongest in the lactation period. According to the theory of "economic phenotype” proposed by Hales & Barker (22) this was to be expected, suggesting the fetal development is sensitive to the nutritional environment. When this is precarious, an adaptive response is triggered to enhance the growth of organs such as the brain, at the expense of other body parts, such as the viscera.
The intestine, as well as other body tissues, undergoes structural and functional changes during development. As age progresses, these changes can be potentiated or reduced depending on the influences of the external environment. The high-fat diet was able to increase the animals’ length of intestine (23). Hounnou et al. (24) concluded that the size of the small intestine is correlated with weight, and not with the height of the individual. In fact, our results showed that the length of small intestine was greater in the HN group which had the highest body weight at weaning.
Regarding the intestine morphometric parameters, there was a reduction in the total height of the mucosa and the villous height in the HH group. In contrast, another study (25) found an increase of the same intestinal parameters in rats subjected to high-fat diet; however, once again, significant methodological differences were found between the investigations. Animals were fed high-fat diet from the third week for the next 8.4 weeks. This makes it difficult to compare the real effect of high-fat diets. Our results reinforce the hypothesis that the high-fat diet used in this study may be harmful to offspring either due its essential fatty acids deficiency (15) or its lack in protein by the reduction in the maternal intake (17).
Based on the presented data, we conclude intake of a cafeteria diet by mothers during pregnancy and/or lactation causes negative consequences for the development of the nervous system of their pups. It seems that a high-fat diet is more deleterious to offspring when consumed during lactation; however, this still needs to be clarified. The cafeteria diet negatively influences intestine morphometry. Further studies are necessary in order to identify the influence of other parameters involved from this kind of cafeteria diet, besides excessive amount of fat.
The authors thank the following Brazilian agencies for financial support: 1-Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Grant # 477456/2010-3 - MCT/CNPq 14/2010); 2-Pro-Reitoria de Pesquisa da UFPE – (PROPESQ – Grants # 23076.023721/2019-28 - Auxílio Pesquisa; # 23076.019425/2019-22 - Edital Qualis A).
The English text of this paper was revised by Pontual Traduções and by Sidney Pratt, Canadian, MAT (The Johns Hopkins University), RSAdip - TESL (Cambridge University).
We have no conflict of interest to declare.
Recibido: 20/03/2021
Aceptado: 03/08/2021