 ,
Maria Zilda de Jesus Catulio1
,
Maria Zilda de Jesus Catulio1  ,
Rávila Graziany Machado de Souza2
,
Rávila Graziany Machado de Souza2  ,
Maria Luiza Ferreira Stringhini1
,
Maria Luiza Ferreira Stringhini1 
Introduction. Crohn's disease (CD) is an inflammatory condition that can affect the entire gastrointestinal tract due to an exacerbated and inadequate immune system response. Objective. This study aimed to conduct a systematic review, through clinical trials, about the use of probiotics in humans with CD. Materials and methods. Research was carried out in the PubMed, Scopus and Science Direct databases using the keywords “Crohn's disease” and “probiotics”. We conducted the review by searching clinical trials published from 2000 to December 2019. Results. Of 2,164 articles found, only nine were considered eligible for this review. The studies investigated patients with CD at different stages of the pathology, and in three studies the potential effect of probiotics in the active phase was observed; in two, in the remission phase; and in four, after intestinal surgery. The sample size of the studies ranged from 11 to 165 individuals and the age of the participants between 5 and 71 years. Gram-positive bacteria were used in six clinical interventions and in two studies yeasts were used. As for the significant results obtained with the treatment with probiotics, in one study there was beneficial clinical effects in patients and, in another, there was an improvement in intestinal permeability. Conclusion. Currently, it is not possible to establish a recommendation for probiotic therapy to control CD due to the few clinical trials with significant results. There is a need for more research on clinical intervention with probiotics in CD to clarify the action, define doses and time of use. Arch Latinoam Nutr 2022; 72(1): 50-59.
Key words: Crohn disease, ulcerative colitis, intestinal mucosa, probiotics.
Introducción. La enfermedad de Crohn (EC) es una afección inflamatoria que puede afectar todo el tracto gastrointestinal debido a una respuesta del sistema inmunitario exacerbada e inadecuada. Objetivo. Realizar una revisión sistemática, a través de ensayos clínicos, sobre el uso de probióticos en humanos con EC. Materiales y métodos. La investigación se realizó en las bases de datos PubMed, Scopus y Science Direct utilizando las palabras clave “enfermedad de Crohn” y “probióticos”. La revisión se hizo en ensayos clínicos publicados desde 2000 hasta diciembre 2019. Resultados. De 2164 artículos encontrados, solo nueve fueron considerados elegibles. Los estudios investigaron pacientes con EC en diferentes etapas de la patología, y en tres estudios se observó el efecto potencial de los probióticos en la fase activa; en dos, en remisión; y en cuatro, tras cirugía intestinal. El tamaño de la muestra fue entre 11 y 165 individuos y la edad entre 5 y 71 años. Se utilizaron bacterias grampositivas en seis intervenciones clínicas y en dos estudios se utilizaron levaduras. En cuanto a los resultados significativos obtenidos con el tratamiento con probióticos, en un estudio hubo efectos clínicos beneficiosos en los pacientes y, en otro, hubo una mejora en la permeabilidad intestinal. Conclusión. Actualmente, no es posible establecer una recomendación de terapia con probióticos para el control de la EC debido a los pocos ensayos clínicos con resultados significativos. Existe la necesidad de más investigación sobre la intervención clínica con probióticos en EC para aclarar la acción, definir dosis y tiempo de uso. Arch Latinoam Nutr 2022; 72(1): 50-59.
Palabras clave: enfermedad de Crohn, colitis ulcerativa, mucosa intestinal, probióticos
https://doi.org/10.37527/2022.72.1.006
Autor para la correspondencia: Maria Luiza Ferreira Stringhini, E-mail: [email protected]
Inflammatory bowel diseases (IBD), of autoimmune origin, are characterized by systemic changes of chronic inflammatory character resulting from excessive and inadequate response of the immune system related to the gastrointestinal tract, among which the most common is Crohn's disease (CD) (1). In a systematic review of population-based study described by Ng et al. (2), published in 2017, the prevalence of CD in Europe ranged from 1.51, in Romania, to 322.0 per 100000 inhabitants in Germany. In Brazil, reliable data on the incidence and prevalence of Crohn’s disease is scarce because IBD is not a mandatory notifiable disease.Thus, public registries and adequate records are limited, resulting in the conduct of regional epidemiological studies (3). In view of this scenario, a study carried out in mid western region of São Paulo State, Brazil, CD prevalence is of 14.8 cases per 100 000 inhabitants (4). In a more recent study, Gasparini et al. (5) estimated that prevalence of CD in the State of São Paulo from January 2012 to December 2015 was 24.3 cases/100 000 inhabitants. Ng et al. (2) noticed that, since 1990, the incidence has been rising in newly industrialized countries in Africa, Asia, and South America, including Brazil with 3.5 per 100 000 person-years, in São Paulo State.
CD affects any segment of the gastrointestinal tract with predominance in the region of the rectum, colon, and ileum in an asymmetric, segmental, and transmural manner, being marked by periods of remissions and activations, affecting mainly the second and third decades of life. It occurs mainly in the inflammatory, fistulous, and fibro-stenosing form with possible extra intestinal evolution of ophthalmological, dermatological and rheumatological involvement. As the main symptoms are abdominal pain, diarrhea, fatigue, fever, intestinal obstruction, nausea, vomiting and weight loss impairing the patient's quality of life (1).
The development of CD has no definite cause, having a multifactorial nature from the interaction of environmental, genetic and immunological factors (6). Among the changeable risk factors is the intestinal microbiota, which has a mutualism relationship with the human being, when in balance. This microbiota is defined as a complex cluster of bacterial colonies that populate the human gastrointestinal tract (7). The amount, type and activity of bacteria that make up these colonies can be modulated by environmental factors such as mode of delivery, health conditions, food, use of antibiotics and smoking, which are also linked to the development of CD, since they alter intestinal homeostasis (8).
From the analysis of the microbiota, it is possible to observe a direct relationship between the colonies in the bowel and the immunological activity of the host. The imbalance of these microorganisms, known as dysbiosis, may generate a pro-inflammatory state with changes in motility and increased intestinal permeability, which leads to the development of metabolic, neoplastic or autoimmune diseases, such as CD (9).
The protective role of the intestinal microbiota in the host is due to the adequate intestinal colonization (10). Thus, possible forms of modulation are described, among which the use of probiotics has been widely investigated. Probiotics are defined as live microorganisms that, when administered in an appropriate concentration, can provide benefit to the consumer, either through local competition, antagonistic action or immunological modulation (10).
Research has been carried out for years in the hope of finding alternatives for prolonging the period of CD remission. Among the clinical trials conducted with the use of probiotics, the results indicate an ambiguous scenario, in which they may decrease the inflammatory response or do not have any beneficial effect for patients with CD (11).
In view of the above and reflecting on this theme, the motivation for this work emerged. Thus, this qualitative systematic review aims to assess the scientific evidence of the effectiveness of therapeutic interventions with probiotics in patients with CD.
This review was conducted, between March to December 2019, using the “Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA) and it is registered in “International Prospective Register of Systematic Reviews” (PROSPERO) under number CRD42020132579.
This qualitative systematic review was carried out in the Medline (PubMed), Scopus and ScienceDirect databases. We conducted the review by searching clinical trials published from 2000 to December 2019. The following descriptors, using Medical Subject Headings (MeSH) “Crohn's disease” and “probiotics” were associated with the Boolean operator “and” to support the search strategy and combine the terms. Reference lists of relevant articles were manually searched identify new studies. Only studies written in English were selected. The search strategy and the total of studies evaluated and selected are shown in Figure 1.
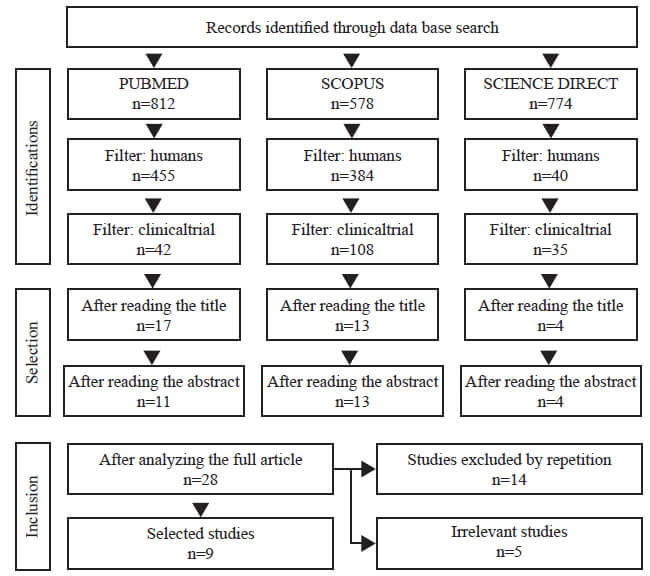
Two independent reviewers performed the review of titles and abstracts. The initial review consisted of a screening of titles and abstract, with subsequent full reading of the work, exclusion of duplicate articles or those which did not meet the proposed inclusion criteria. Discrepancies were discussed through consultation with a third reviewer. Randomised controlled trials that compared probiotics with placebo or any other non-probiotic intervention in humans, children, adolescents and adults, original works who analyzed the use of probiotics in modulating the intestinal microbiota or who evaluated the efficacy and safety of probiotics in inducing remission in Crohn's disease were considered. The following articles were excluded: animal studies, case reports, reviews, and editorials.
The following variables were extracted from each study: first author; year of publication; goals; design; sample size; strains and colony forming units (CFU), time of intervention and results related to clinical, biochemical, and immunological improvement of patients.
This qualitative systematic review selected nine articles that met the previously established criteria (11-19). All articles were randomized, double-blind and placebo-controlled, with follow-up time between 3 and 24 months, of which five were multicentric (11-15). There was a regular temporal distribution of articles related to clinical intervention with the use of probiotics from the year 2000 to 2015, with a focus in the period of CD remission, through the control of signs and symptoms intrinsic to the pathology. Between 2015 to 2019 no clinical intervention was found that comply with the inclusion criteria.
The studies investigated patients with CD at different stages of the disease, and in two studies the potential effect of probiotics in the active phase (16,17) was evaluated, three in the remission phase (11,12,18) and in four studies, patients after intestinal surgery (13-15,19) were analyzed. No studies were found relating probiotics to modulation of the intestinal microbiota. The sample size of the studies ranged from 11 to 165 individuals and the age of the participants ranged from 5 to 71 years.
Gram-positive bacteria were used in six clinical interventions and in three studies yeasts were used (Table 1). Lactobacillus rhamnosus stripe GG were used in three clinical trials (12,16,19); Lactobacillus johnsonii LA1(13,14) in two; and in one there was an association of eight different bacteria (Lactobacillus – L. pracasei DSM24733, L. plantarum DSM24730, L. acidophilus DSM24735 and L. delbrueckiisubsp bulgaricus DSM24734; Bifidobacterium – B. longum DSM24736, B. breve DSM24732 and B. infantis DSM247377 and Streptococcus salivarius subsp thermophilus DSM24731)(15). The authors used Saccharomyces boulardii as probiotic in three research papers (11,17,18). In the research by Bousvaros et al. (12) inulin was also used, together with the probiotic.
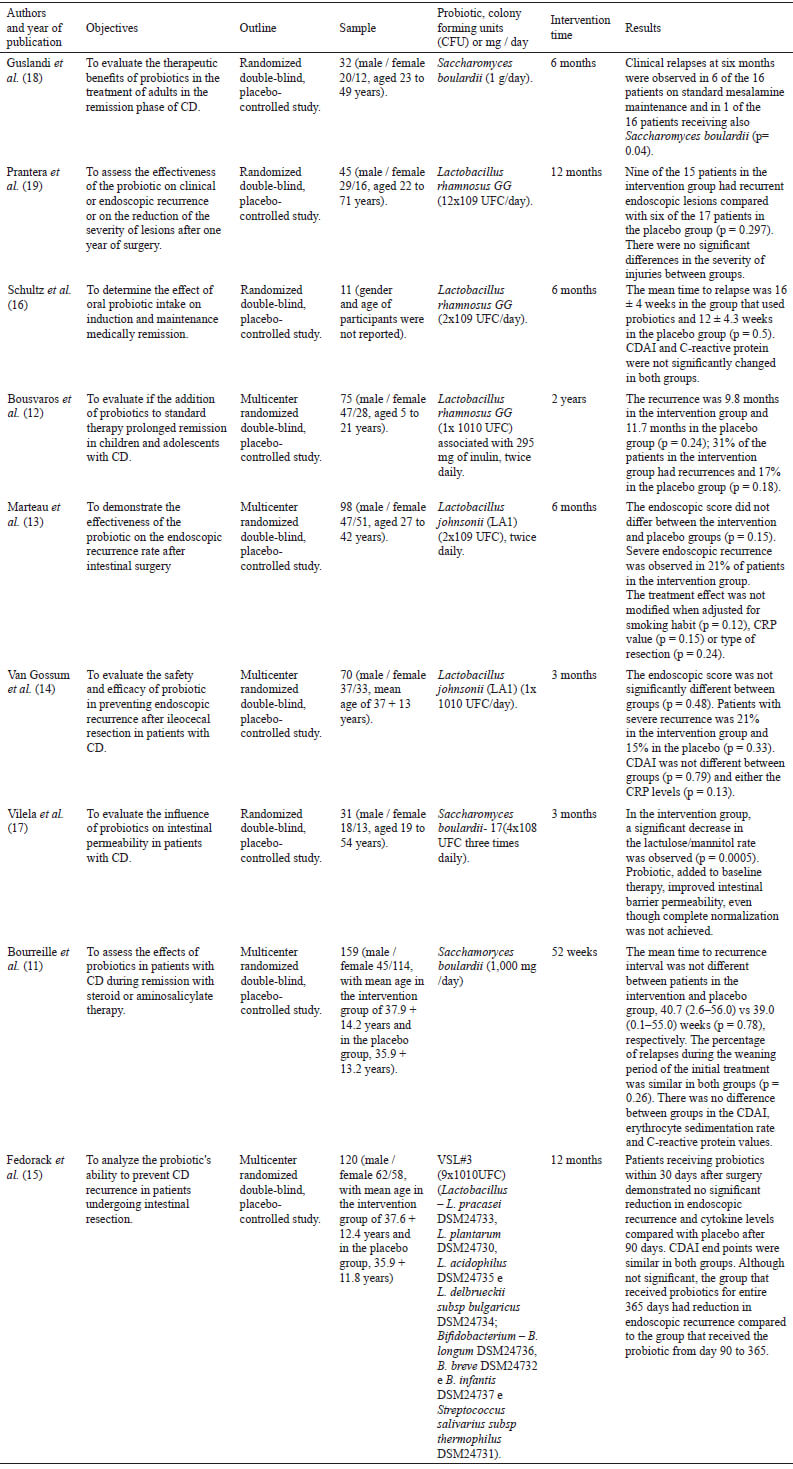
As for the positive results obtained in therapy with probiotics, it was found that in the study by Guslandi et al. (18), there was a significant reduction in clinical relapses, after six months, on patients receiving Saccharomyces boulardii (1 g / day) together with mesalamine (2 g/d) (p=0,04). In another clinical intervention carried out by Vilela et al. (17), the same yeast contributed to a significant reduction in the lactulose / mannitol ratio and increased the function of the intestinal barrier. Other clinical trials in this systematic review did not find significant results, especially in the rate of recurrences, among the groups that used probiotics and placebo (Table 1).
This review included nine clinical trials conducted between the years 2000-2019, aiming to analyze the role of probiotics in the treatment of CD. The results of seven of these studies attested the ineffectiveness of the probiotic therapy adopted to prolong remissions or improve signs and symptoms. It was possible to find points of convergence between the studies by Prantera et al. (19) and Schultz et al.(16), whose research field was the use of the probiotic strain Lactobacillus GG in the gastrointestinal tract of adult CD patients, but with different time, concentration, and association with other drug therapies. However, such factors did not influence the result found, since in both studies there was no prolongation of remission and / or improvement of injuries when compared to the control group.
Still concerning the investigation of the efficacy of the use of Lactobacillus GG in CD, a multicenter study was carried out in 2005 with young people and children in remission of the disease for at least two months using amino salicylates, azathioprine or corticosteroids in low doses. However, the results obtained also indicated that the mean time to recurrence was similar in both the intervention and control groups (12).
As for the studies that analyzed the modulation capacity of the intestinal microbiota using Lactobacillus GG, it appears that none reached its objective in proving the probiotic's effectiveness in prolonging the remission time of CD, in different stages of the disease. In the study by Prantera et al. (19), for example, it would be necessary to consider the largest number of smokers in the intervention group, but without statistical difference in relation to the placebo group. The same was verified in the population studied by Marteau et al. (13), in which there was a greater presence of smokers or ex-smokers in the group receiving the probiotic. It is noteworthy that in both studies, smoking was not considered an exclusion factor to participate in the clinical trial.
It is known that smoking is one of the environmental factors responsible for the increase in immunosuppressants, being able to alter endothelial function with impairment of mucosal integrity and to modify the intestinal microbiota, in addition to increasing oxidative stress through the activation of monocytes and macrophages, which release pro-inflammatory cytokines such as IL-6 and TNF-α (20). Thus, the inclusion of smokers in the study group could negatively influence the results regarding the effect of using probiotics in the studies by Prantera et al. (19) and Marteau et al. (13).
In the work by Bousvaros et al. (12), the presence of inulin in the capsules of both the intervention group and the placebo group could contribute to significant results related to the recurrence of CD, which were not verified. Inulin is a polymer of fructose present in many vegetables. As a functional ingredient, it is a prebiotic capable of influencing the intestinal microbiome, selectively promoting the growth of bacteria native to the digestive tract by producing a range of short-chain fatty acids that lower the overall pH of the digestive system, preventing colonization by pathogens (21).
In the essay by Schultz et al. (16), the small sample size (n = 11) and the short intervention period (six months) are the warning points. The authors reported that, due to the low inclusion rates of patients and the negative effects of the probiotic on CD remission, the study was early interrupted. Another difficulty observed in this clinical trial is related to the methodology, which does not clarify the placebo composition. In the end, they concluded that Lactobacillus rhamnosus GG did not induce nor allow the induction of medications (corticosteroids) to improve the CD Activity Index.
Guslandi et al. (18), Vilela et al. (17) and Bourreile et al. (11) had as common premise the effectiveness analysis of the yeast S. boulardii in the treatment and maintenance of CD remission; however, in the methodology they used time, number of participants, probiotic concentration and association with different drug therapies. The authors possibly used S. boulardii because it is a probiotic yeast with beneficial effects on the intestinal epithelial barrier and on the digestive immune system, since it acts by inhibiting the growth of pathogenic bacteria, as already discussed in the literature (22).
Guslandi et al. (18) found a possible beneficial association of probiotic therapy. However, unlike the other studies, this one associated the yeast S. boulardii (1g / day) with mesalamine (2g / day) and, after six months, the remission was measured through a reduction in the Crohn’s disease activity index (CDAI) (data not shown). The disease is in remission when the CDAI is less than 150; mild to moderate when it oscillates between 150 and 219; moderate to severe between 220 and 450; and severe or fulminating when the values are greater than 450 (1). Another discussion of this study is that, in the intervention group, the dose of mesalazine (2 g/d) was lower than in the placebo group (3 g/d) and this fact introduced an uncontrolled variable that could modify the results of the intervention group.
Vilela et al. (17) observed an improvement in intestinal permeability when associating S. boulardii with standard therapy (mesalamine, azathioprine, prednisone, metronidazole and thalidomide) used by patients; however, differently from other studies, this one evaluated the improvement by using the lactulose/mannitol ratio test. This analysis consists of the administration of lactulose and mannitol, through oral overload, and the determination of both substances in the urine, by high-performance liquid chromatography, informing the percentage of absorption and, consequently, intestinal integrity and absorptive function (23).
Mannitol is a monosaccharide that, in normal situations, is absorbed between 5 and 30 %. Its analysis informs the degree of absorption of small molecules (<0.4 nm) by transcellular route. Lactulose, on the other hand, is a disaccharide that must be absorbed at levels below 0.5 % and its determination indicates the degree of absorption of large molecules (> 0.5-0.6 nm) by paracellular route. So, an increase in lactulose recovery reflects an increase in paracellular permeability (between cells), which allows toxins, antigens, peptides or even macromolecules to cross the intestinal barrier (23).
The calculation of the larger marker/smaller marker ratio reduces individual variation caused by factors such as gastric emptying, intestinal transit, and difficulty in collecting urine and increases the accuracy in the evaluation of intestinal permeability (23). Also, lactulose/mannitol ratio permits a more precise and sensitive monitoring of the therapeutic response than clinical observation in patients with CD, since these tests can detect repercussionsof alterations intimately associated with the inflammatory processes present in the disease (17).
Continuing the analysis of the effectiveness of S. boulardii, the study by Bourreile et al. (11) evaluated patients after treatment with corticosteroids (systemic or topical) and salicylates, so that it was not possible to notice positive results related to this probiotic therapy in patients. According to Veauthier and Hornecker (1), corticotherapy reduces inflammation through the glucocorticoid receptor. Within the cell, cortisol, in combination with the receptor, is associated with transcription factors, such as the nuclear factor kB, with a reduction in the production of inflammatory proteins. This factor was also elucidated by Lamb et al. (24), who established corticosteroids and amino salicylates as effective drug therapies in the treatment of IBD, such as CD.
In the same study by Bourreile et al. (11), the probiotic was administered to patients with different smoking habits. In this case, the habit of not smoking favored the results of patients with CD who received S. boulardii to the detriment of those who received the placebo, with no difference in the time of relapse of the disease between smokers and ex-smokers even with the administration of the probiotic.
Thus, it is observed that most clinical trials in this review failed to establish a cause-effect relationship between the attenuation and remission of CD caused by the administration of the probiotic. Among other factors, the use of various drugs to control the disease, the difference in location and disease activity in patients allocated to each group, as well as the limited number of individuals in the intervention and control group may have affected the results.
In addition to these aspects, the studies carried out did not observed changes in increasing the diversity and abundance of beneficial microbial species using probiotics, which requires genetic sequencing for the complete determination of the intestinal microbiota. It is known that this microbiota is extremely important for the maintenance of the health of the host; however, so far it has not been described whether changes in the intestinal flora would be a consequence or cause of diseases and the mechanisms of this modulating role still need to be better understanding (25).
Another factor to be considered is that in none of these surveys there was a report on the participants' diet. According to Reddavide et al. (26), foods such as vegetables and fruits, abundant in fibers and micronutrients (antioxidants and anti-inflammatories), help to reduce inflammation in the mucosa and maintain the function of the intestinal barrier, helping to delay symptoms CD and other IBD. According to the current guidelines, in the treatment of IBD the food groups mentioned by the author are recommended and may decreased intestinal permeability (27). It is known that there is no diet that can be generally recommended to promote remission in IBD patients with active disease and no specific diet needs to be routinely followed during remission phases of CD (27). However, it would be important to control patients' diet in researches.
We are still far from fully translating this research into clinical and therapeutic treatment applied to IBD. Studies that relate probiotic therapy with the purpose of increasing the time of remission of CD have as limiting factors the lack of control of the participants' diet; the use of Saccharomyces boulardii and Lactobacillus as probiotics only and the results cannot be extrapolated to other strains or dosages; the different numbers of colony forming units and treatment time. In addition, since the CDAI is not, currently, considered a good parameter for measuring intestinal inflammation, we suggest incorporating other parameters such as endoscopy, magnetic resonance imaging, US Doppler and calprotectin, among others, in future studies to characterize possible changes in the mucosa intestinal by probiotics. All authors agree with the need for other clinical trials, especially to clarify the mechanisms of action of probiotics and the factors of the digestive ecosystem that can influence this performance. Such clarifications should allow to optimize the use of probiotics in the treatment of patients with IBD. The available evidence is very uncertain about the efficacy of probiotics for induction of remission in Crohn's disease.
Despite the possible evidence related to probiotic therapy in the remission of CD, only two intervention studies carried out, to date, prove the effectiveness of its use in remission or in the clinical improvement. The data are still insufficient and the results confusing. These facts have led to a failure to define the appropriate probiotic treatment for DC suggesting that well-designed randomized control trials are needed for future research. Furthermore, a better understanding of the gut microbiome will also determine the role of probiotics as therapeutic agents in the management of IBD.
We declare that there is no conflict of interest.
Recibido: 12/05/2021
Aceptado: 10/12/2021