 ,
Lilia Leticia Méndez-Lagunas1
,
Lilia Leticia Méndez-Lagunas1  ,
Juan Rodríguez-Ramírez1
,
Juan Rodríguez-Ramírez1  ,
Sadoth Sandoval-Torres1
,
Sadoth Sandoval-Torres1  ,
Laura Victoria Aquino-González1
,
Laura Victoria Aquino-González1  ,
Luis Gerardo Barriada-Bernal2
,
Luis Gerardo Barriada-Bernal2 
Introduction: The use of vegetable proteins as ingredients in food systems is based on their functional properties. The water and oil holding capacity, foaming, and emulsifying capacity/stability, and antioxidant assay of the protein fractions - albumins, globulins 7S/11S, glutelins and prolamins - isolated from Leucaena seed were evaluated. Objective: The objective of this study was to evaluate the functional properties and antioxidant capacity of the concentrate and protein fractions of ripe Leucaena spp. seeds. Materials and methods: Ripe Leucaena seeds were collected and evaluated in Oaxaca, Mexico (16°59’21’’N 96°43’26’’O) during the months of February-April 2021.The protein concentrate was isolated by isoelectric precipitation (pH=9, pH=4). The albumins, globulins, glutelins and prolamins were isolated based on their solubility properties in different extracting solutions. Results: Glutelins constituted the main protein fraction (75.88%). Prolamins were not found. The glutelins fractions showed the highest oil holding capacity (0.93±0.08 mL g-1). The albumins fraction had the highest water holding capacity (2.53±0.15 mL g-1), foaming capacity and foam stability (71.83±1.26 % and 70.00±0.00%, respectively) and antioxidant capacity (18.09±0.88%). The globulins exhibited the highest emulsifying capacity and emulsion stability (56.83±1.76% and 55.67±1.20%, respectively). Conclusions: The concentrate and protein fraction of Leucaena seeds showed different techno-functional and antioxidant properties of interest for the food industry, like those showed by other commercial vegetable proteins. Arch Latinoam Nutr 2022; 72(3): 196-204.
Key words: Leucaena spp., antioxidant capacity, protein fractions, techno-functional properties.
Introducción: El uso de proteínas vegetales como ingredientes en sistemas alimentarios se basa en sus propiedades funcionales. Se evaluó la capacidad de retención de agua y aceite, la capacidad/estabilidad espumante y emulsionante y el ensayo antioxidante de las fracciones proteicas -albúminas, globulinas 7S/11S, glutelinas y prolaminas- aisladas de las semillas de Leucaena. Objetivo: El objetivo de este estudio fue evaluar las propiedades funcionales y la capacidad antioxidante del concentrado y las fracciones proteicas de las semillas maduras de Leucaena spp. Materiales y métodos: Las semillas maduras de Leucaena fueron recolectadas y evaluadas en Oaxaca, México (16°59’21’’N 96°43’26’’O) durante los meses de febrero-abril del año 2021. Se usó harina de Leucaena desgrasada para la preparación de las fracciones proteicas. El concentrado proteico se aisló por precipitación isoeléctrica (pH=9, pH=4). Las albúminas, globulinas, glutelinas y prolaminas se aislaron en función de sus propiedades de solubilidad en diferentes soluciones de extracción. Resultados: Las glutelinas constituyeron la principal fracción proteica (75,88%). No se encontraron prolaminas. La fracción de glutelinas mostró la mayor capacidad de retención de aceite (0.93±0,08 mL g-1). La fracción de albúminas presentó la mayor capacidad de retención de agua (2,53±0,15 mL g-1), capacidad espumante y estabilidad de la espuma (71,83±1,26% y 70,00±0,00%, respectivamente) y capacidad antioxidante (18,09±0,88%). Las globulinas mostraron la mayor capacidad emulsionante y estabilidad de la emulsión (56,83±1,76 y 55,67±1,20%, respectivamente). Conclusiones: El concentrado y las fracciones proteicas de las semillas de Leucaena mostraron diferentes propiedades tecno-funcionales y antioxidantes de interés para la industria alimentaria, similares a los reportados por diversas proteínas vegetales comerciales. Arch Latinoam Nutr 2022; 72(3): 196-204.
Palabras clave: Leucaena spp., capacidad antioxidante, fracciones proteicas, propiedades tecno-funcionales.
https://doi.org/10.37527/2022.72.3.005
Nowadays the vegetable proteins are intensively used in human and animal food formulations as a functional ingredient (1). Seed proteins of legumes can be used to improve nutritional quality, techno-functionality and the nutraceutical properties in food formulations (2). Most research and development efforts have been conducted in legume seeds such as soybeans, peas and common beans (3), but for other non-commercial legumes seeds such as Leucaena, research has been minimal (4), this could be due to the fact that the Leucaena plant is mainly used in the feeding of cattle as important part of the protein intake(5), and the presence of anti-nutritional factors, such as tannins and mimosine, minimize its use in the industry (6), so it is necessary to generate strategies to reduce risks and maximize its nutritional and functional advantages.
Leucaena is a fast-growing leguminous tree that belongs to the Fabaceae (Leguminosae) family. It is a legume with multiple blooms events in a year end highly adaptable to multiple environmental/soil conditions (7). Leucaena is used mainly for fodder and timber (8,9), and the humans have also eaten its seeds (4). The protein fraction in seeds of Leucaena has been reported in a range of 24.50% to 46.00% per seeds dry weight (4,10). It has been indicated that Leucaena seed flour has water and oil absorption capacity, but no studies have been conducted on the protein fraction (4).
The aim of this study was to identify the techno functional properties of the protein fractions of Leucaena seeds (Leucaena spp.)
Ripe Leucaena spp. seeds were collected in Oaxaca, Mexico (16°59’21’’N 96°43’26’’O) during the months of February-April 2021.
Ripe seeds were removed from their pods and cleaned in a commercial solution of 5% (v/v) sodium hypochlorite. Subsequently, the seeds were subjected to a convective drying process (40°C) until they reached a humidity of less than 10%. Seeds were reduced to particulate material less than 0.149 mm and stored at 25°C.
The total protein fraction was obtained according to the methodology of Ohara et al. (2020)(11) with some modifications. The flour was suspended in distilled water 1:10 (w/v); the pericarp was removed by centrifugation (453 x g). The pH of the suspension was fixed at 9.0 by adding 0.1N of sodium hydroxide. The suspension was shaken for 1 hour at room temperature (25°C). The non-protein fraction was removed by centrifugation (453 x g). The supernatant was recovered, and the pH of the solution was adjusted to 4.0 with hydrochloric acid 1N (isoelectric protein point). The precipitated protein fraction was recovered by centrifugation (453 x g) and stored at 25°C.
The methodology of Maldonado-Cervantes et al. (2010) (12) with certain modifications was used to isolate the albumins, the 7S/11S globulins, the prolamins and the glutelins.
Leucaena seed protein fractions were extracted from defatted flour. The extraction was performed sequentially. Albumins were extracted with distilled water; the flour/water (1:10, w/v) suspension was stirred for 30 min and centrifuged at 453 x g for 20 min. The supernatant was collected and kept at −5 °C until used. The pellet was dissolved in 0.1 M NaCl, 10 mM K2HPO4 at pH 7.5, and 1 mM EDTA; it was stirred and centrifuged as described above, and the supernatant was considered to be the 7S globulin fraction. The pellet was then used for extraction of the 11S globulin fraction; after the pellet was dissolved in 0.8 M NaCl, 10 mM K2HPO4 at pH 7.5, and 1 mM EDTA, the mixture was stirred and centrifuged as described above. The resulting pellet was dissolved in 50% methanol; it was stirred and centrifuged as described above, the supernatant was the prolamin fraction. The glutelin fraction was extracted from the last pellet with 0.1 M NaOH. The protein fractions were dehydrated in a convection oven at 60°C to reach 10% humidity and stored at 25°C.
The protein concentration was determined by the 955.04 AOAC method (13), using the nitrogen conversion factor of 5.7.
Water and oil holding capacity were evaluated according to the methodology of Wang and Kinsella (1976)(14), with some modifications.
Distilled water (5 mL) was added to 0.50 g of each protein fraction evaluated in a graduated volumetric vessel. The initial volume occupied only by the distilled water was registered. The sample was dispersed by ultrasonic pulses (44 KHz, 200W) for one minute. The resulting suspension was vortexed for 30 minutes at room temperature (25°C) and centrifuged (453 x g for 25 min). The volume of water below the suspension formation line was recorded and compared with the initial volume (distilled water level). The volume of water absorbed was expressed as milliliters of water absorbed per gram of dry protein fraction (mL g-1).
Virgin olive oil (3mL) was added to 0.50 g of the sample in a graduated volumetric vessel. The initial volume occupied by the vegetable oil was registered. The sample was dispersed by ultrasonic pulses (44 KHz,200W) for one minute. The resulting suspension was vortexed for 30 minutes at room temperature (25°C) and centrifuged (453 x g for 25 min). The volume of oil below the suspension formation line was recorded and compared with the initial volume. The volume of free oil absorbed was expressed in milliliters of oil absorbed per gram of dry protein fraction (mL g-1).
Emulsifying properties were evaluated according to the methodology of Chaparro et al. (2014)(15) with some modifications. In a graduated volumetric vessel, 0.70 g of the sample was dispersed in 10 mL of distilled water. A homogeneous suspension was formed by stirring using low-intensity ultrasonic pulses (44 KHz, 200W). Ten milliliters of virgin olive oil were added to the homogeneous suspension. The formation of an emulsion was promoted for one minute by vortex agitation. The emulsion was divided equally in two graduated volumetric containers of equal characteristics. The first vessel was centrifuged at 453 x g for 5 minutes. The volume of the resulting emulsion interface was quantified. The emulsifying capacity of the sample (EC) was calculated using Equation 1. The second volumetric vessel was heated in a water bath for 30 minutes at 80°C;the temperature was then lowered in an ice bath until 15°C was reached. It was then centrifuged at 453 x g for 5 minutes, and the volume of the resulting emulsion interface was quantified. The emulsion stability (ES) was calculated using Equation 2.
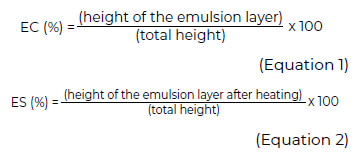
Foaming properties were evaluated according to the methodology of Kumar et al. (2014)(16) with some modifications. A suspension was formed in a graduated volumetric vessel with 0.5 g of the sample and 30 mL of distilled water. The suspension was vortexed for 5 minutes at room temperature (25°C) to immediately register the volume of foam generated. Foaming capacity (FC) was calculated using Equation 3. To determine foaming stability (FS), the decrease in foam volume after 1 hour of resting at room temperature was recorded. The stability of foam formed was calculated using Equation 4.

Antioxidant activity was determined using the methodology of Brad-Williams et al. (1995) (17) with some modifications. The reaction mixture consisted in a 100 mL of 2.50 mg mL-1 of a solution of the protein fraction diluted in a methanol-water (80% v/v) solution. Then, 2.90 mL of 2, 2-difenil- 1-picrylhydrazyl solution, DPPH* (0.039 mg mL-1) were added. The reaction mixture was vortexed and stored for 30 minutes in the dark at 25°C. The reaction mixture was evaluated by spectrophotometry at a wavelength of 517 nm. The antioxidant capacity (AC) was determined using Equation 5.

Extraction, characterization, and evaluation assays were performed in triplicate. A onefactor analysis of variance and Tukey's test (p=0.05) were performed with Statistica 7.0 software.
The protein content of Leucaena seed flour was 35.35±0.49 g 100 gdb-1. The protein content of the concentrate protein fraction was 72.42±1.21 g 100 gdb-1.
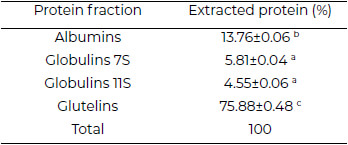
Glutelins were the highest fraction in the protein isolated from Leucaena seeds, followed by albumins (Table 1). No prolamin fraction was registered.
The protein concentrate showed a water holding capacity of 1.30 mL gdb-1, whereas the albumin protein fraction exhibited the highest water holding capacity: 2.53±0.15 mL g-1.
The protein concentrate showed an oil holding value of 0.33 mL gdb-1. The glutelin protein fraction reached the highest value of oil holding capacity: 93 mL g-1.
The emulsifying capacity values of the Leucaena protein concentrate (Table 2) were higher than those reported by Rosida et al. (2016) (18), from 47.7 to 50.20%. The protein concentrate, the globulins 11S and the glutelins have the highest emulsifying values of the protein fractions evaluated.
The foaming activity of the protein concentrate(Table 2) was similar to the results reported by Wani et al. (2015)(19) for the protein fraction from various common bean (Phaseolus vulgaris) varieties (76-102%). Rosida et al. (2016)(18) reported a foaming activity of 9.8% for Leucaena leucocephala protein concentrates. The albumin protein fraction showed the highest values for foaming capacity and foaming stability with respect to the other protein fractions.
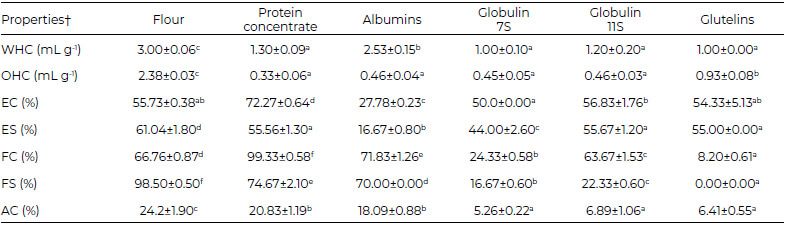
Whole flour has the highest antioxidant capacity (Table 2). The albumin fraction had the better antioxidant capacity of all protein fractions evaluated.
The protein content of Leucaena seed whole flour (35.35±0.49%) was higher than the average protein content from other commercial legumes,such as common beans, 17.5-28.7 %; lentils, 21.7-31.4 %; and chickpeas 12.4-30.6 % (20). The protein profile of the Leucaena seed whole seed is then similar to other seeds of dicotyledonous legumes, such as common beans, where the fraction of globulins and albumins is considered the highest protein fraction (21). In monocotyledonous, the glutelin fraction is the principal reserve protein and it is the highest protein fraction in some cereals such as rice (22). The albumins fraction from legumes seeds is reserve proteins fraction (23) or proteins fraction with a specific metabolic role, such as the degradation of reserve proteins during germination (24).
The protein concentrate had a water holding capacity of 1.30 mL gdb-1, similar to values reported by Boye et al. (2010)(26) for legume protein concentrates of peas, lentils and chickpeas in a range of 0.60-2.70 mL gdb-1. Rosida et al. (2016)(18) reported for Leucaena seed isolates water holding values of 3.12-3.22 mL gdb-1. The albumin fraction water holding capacity was 2.53 mL gdb-1; higher than the reported by Sánchez-Mendoza et al. (2017) (27), for Inga Paterno, 1.73 mL gdb-1. The water holding capacity (WHC) of a food matrix or a protein is a physical property of their three dimensional structure that prevents its inner water to be released by an external force, such as pressing, centrifugation, or heating (28). According to Kinsella and Whitehead (1989)(29), at least six mechanisms of water protein association, referred to the water holding property, can be distinguished: 1) structural water; 2) hydrophobic hydration; 3) monolayer water; 4) un-freezable water; 5) capillary water and 6) hydrodynamic hydration water and the amount of water associated with a protein structure depends on factors, such as its amino acid composition, number of exposed polar groups, surface hydrophobicity, pH value, ionic composition and strength, temperature, and concentration.
In a food matrix, protein-bound internal water molecules do not represent a significant quantity of the total water amount (e.g., in meat, they represent about 5 % of the total water amount). The rest of the water molecules are entrapped to another food matrix component (e.g. oils, starch and other solutes)to the physical structure of the matrix or they are free (30). The analysis of the water holding capacity is fundamental in developing a food matrix texture, especially in minced meat products and baked dough (31). Protein ingredients with a high WHC increase their viscous behavior and can be used in soups, sauces, doughs and baked goods (32). Protein ingredients with very low WHC may dehydrate other ingredients in a food system or can be more sensitive to storage humidity (33).
The protein concentrate showed an oil holding capacity of 0.33 mL gdb-1, which is lower than that reported by Rosida et al. (2016), 1.20 g gdb-1, for Leucaena leucocephala. The protein fractions of albumins and 7S and 11S globulins, report average values of 0.45 mL gdb-1, while Sanchez Mendoza et al. (2017)(27) reports for Inga paterno higher comparative values, 0.92 and 3.8 mL gdb-1, respectively. The OHC behavior is affected by several factors like protein source, size and protein concentration and the no polar amino acidlipid interactions (the amino acid bond to the hydrocarbon side chains of the oil). This property is very important in flavor retention and texture in the preparation of fried foods, baked goods and several applications to improve the batter, flavor and emulsions food characteristics (23, 24). A high OHC of the protein fraction in a food matrix decreases the development of oxidative rancidity and increases the stability of products during storage (20).
The emulsifying capacity values of the Leucaena protein concentrate (72.27%) were higher than those reported by Rosida et al. (2016)(18) for Leucaena leucocephala protein concentrates (47.7-50.20%), as well as those reported by Wani et al. (2014) (19), 15.71 - 48.92 %, for common bean protein concentrates. The albumin fraction has emulsifying capacity values of 27.78 % and the 7S and 11S globulin fraction, values of 50 and 56.83% respectively; these values are higher than those reported by Sánchez-Mendoza et al. (2017)(27) for Inga paterno albumins and globulins, with ranges between 1-3% and 4-40%, respectively. This capacity was influenced by pH, being favored when the medium is alkalinized. Proteins are amphiphilic molecules that facilitate the formation of the emulsion, reducing the surface tension between the non-polar and polar phases. Their ability to be adsorbed at the interface of oil or air droplets allows for the development of stable films and dispersions, thus acting as emulsifiers. The dispersion of oil droplets in an aqueous medium or the surrounding of air cells with a film leads to the formation of emulsions and foams, respectively (35). The emulsifying capacity is directly influenced by several intrinsic protein attributes such as molecular weight, conformational stability, water solubility, amino acid composition and the hydrophobicity-hydrophilicity ratio at the surface, as well as by external parameters such as pH, temperature and ionic strength (36). This property is of great importance in salad dressings and meat products as indicated by Betancur-Ancona et al. (2004) (37). In foods, emulsions are of the oil-in-water type, such as milk and mayonnaise, or water-in-oil type such as butter and margarine (38).
The foaming activity of the protein concentrate (99.33±0.58%) was in accordance with the results reported by Wani et al. (19), for the protein fraction from various common bean varieties (76-102%). By contrast, Rosida et al. (2016)(18) reported a foaming activity of 9.8% for Leucaena leucocephala protein concentrates. In relation to fractions, the albumin fraction exhibited the highest values of foaming capacity and foaming stability (71.83±1.26%, 70.00±0.00%, respectively). It has been reported that albumins, globulins and defatted bean meal have shown above 65%, Lawal et al. (2005) (39), while values of 84.6 and 42.4% were reported for albumins and globulins obtained from Gingko biloba, respectively, under basic conditions (pH between 8 and 9)(40). Vani and Zayas (1995) (28) indicate that the expansion and stability of the foam are directly correlated with the protein concentration. The proteins change the viscosity of the continuous phase and promote foam expansion and stability. In a protein foaming system, three sequential stages are involved: 1) initially, the soluble globular proteins diffuse to the air-water interface; 2) the protein concentrates and the surface tension is reduced; 3) a concurrent reorientation of the polypeptides forms a continuous film (41). This property is important in the production of beer, bread, whipped cream, ice cream, meringues, candies, icings, fudges, nougats, etc. (42).
Dueñas et al. (2007) (43) studied the antioxidant capacity present in the lentil testa and cotyledon and observed that this capacity was mainly due to flavonoid compounds. Bravo-Delgado et al. (2019) (4) conducted DPPH* assays on hot air-dried Leucaena flour (60°C/12 hours) and reported a 90.70% of inhibition; this value contrasts with the one obtained during this study, 20.83±1.19 %. It could be explained by the variety, the geographical location, the cultural practices of the region and the type of drying used (44). Román-Cortés et al. (2014) (45) reported values of 91.70% inhibition of fresh Leucaena seed flour. In this case, the degree of seed maturity could influence the polyphenols content and the antioxidant capacity of the Leucaena seed. Boukhanouf et al. (2016) (46) found a decrease in polyphenols content of 55.1% between mature and immature pods of Vicia faba.
The evaluation of the functional properties of the flour, concentrate and protein fractions derived from the Leucaena seed, allows identifying its potential as a food matrix functional ingredient. The main protein fraction of the Leucaena seed was glutelins (the highest oil holding capacity). The albumins had the highest water holding and foaming capacities and stability, whereas the globulins exhibited the highest emulsifying capacity and stability.
I would like to thank the Consejo Nacional de Ciencia y Tecnologia (CONACyT) and the Instituto Politécnico Nacional (IPN) for the facilities and financial support provided for this manuscript.
The authors report the non-existence of any conflict of interest with any public institution, private corporation/company, or another research group.
Recibido: 24/05/2022
Aceptado: 13/06/2022