 ,
María Cristina Ortíz León1
,
María Cristina Ortíz León1  ,
Héctor Hernández-Gutierrez2
,
Héctor Hernández-Gutierrez2  ,
Roberto A. Bahena-Cerón3
,
Roberto A. Bahena-Cerón3  ,
Aidé Miranda-Reza4,
José A. Marin-Carmona5
,
Aidé Miranda-Reza4,
José A. Marin-Carmona5  ,
Edit Rodríguez-Romero1
,
Edit Rodríguez-Romero1  ,
Silvia I. Mora-Herrera6
,
Silvia I. Mora-Herrera6  ,
Jonathan Garcia-Roman7
,
Jonathan Garcia-Roman7  ,
Julio I. Peréz-Carreón8
,
Julio I. Peréz-Carreón8  ,
Eduardo Rivadeneyra-Domínguez3
,
Eduardo Rivadeneyra-Domínguez3  ,
Gabriel Riande-Juárez1,
and Rebeca Garcia-Roman1*
,
Gabriel Riande-Juárez1,
and Rebeca Garcia-Roman1*  .
.
Introduction. Non-Alcoholic Fatty Liver disease (NAFLD) can lead to Non Alcoholic steatohepatitis (NASH), cirrhosis, and liver cancer. The treatment for NAFLD involves modification of caloric intake and physical activity. NAFLD has a pro-oxidant nature; therefore, it is logical to suppose that the antioxidant methionine can be used as a treatment for this disease. Aim. This study aimed to evaluate the effect of high-methionine dietary therapy on patients with NAFLD. Materials and methods. A randomized clinical study was conducted over three months. In this study, 121 NAFLD patients participated, and the age of the participants was ≥ 20 years (experimental group included 56 and control group 65), all of whom were randomized and matched by sex, recluted from the ISSSTE hospital in Xalapa, Mexico. The patients were instructed to consume food to cover the recommended methionine daily doses, and the daily amount consumed was calculated. Methionine effect was measured as NAFLD regression and quality of life improvement. Results. Nutritional therapy induced NAFLD regression and diminished central fat accumulation, blood pressure, and the fatty liver index. Some parameters, such as liver enzymes, did not changed. The quality of life of patients improved after treatment. Conclusions. In this study, we show a hepatoprotective effect induced only in three months of chances in the diet, thus, a longer diet may generate more relevant benefits in the resistant parameters of our study. Arch Latinoam Nutr 2023; 73(2): 122-134.
Keywords: methionine, NAFLD reversal, and nutrition therapy.
Introducción. La enfermedad del hígado graso no alcohólico (NAFLD) puede conducir a la esteatohepatitis no alcohólica (NASH), la cirrosis y el cáncer de hígado. El tratamiento para NAFLD es la modificación de la ingesta calórica y la actividad física. Debido a que NAFLD tiene una naturaleza pro-oxidante; es lógico suponer que el antioxidante metionina puede utilizarse en el tratamiento de esta enfermedad. Objetivo. el presente trabajo evaluó el papel de la terapia nutricional con alimentos ricos en metioninaen pacientes con NAFLD. Materiales y Métodos. Se realizó un ensayo clínico aleatorizado durante tres meses. Participaron en el estudio 121 pacientes con NAFLD con edad ≥ 20 años (56 en el grupo experimental y 65 en el control), todos aleatorizados y pareados por sexo, reclutados de la Clínica Hospital ISSTE en la ciudad de Xalapa, México, en el año 2015. Se instruyó a los pacientes en consumir los alimentos hasta completar la dosis diaria recomendada de metioninay se calculó la cantidad diaria consumida. Su efecto se midió como la regresión de NAFLD y la mejora de la calidad de vida. Resultados. La terapia nutricional retrocedió NAFLD; disminuyó la acumulación de grasa central, la presión arterial y el índice de hígado graso. Algunos parámetros, como las enzimas de la función hepática, no se modificaron con el tratamiento. Otro parámetro fue la mejora de la calidad de vida de los pacientes tratados. Conclusiones. En este trabajo mostramos un impacto hepatoprotector producido con tan solo tres meses de cambios en la dieta, por lo que una dieta más prolongada podría generar beneficios aún más significativos en los parámetros resistentes en nuestro protocolo. Arch Latinoam Nutr 2023; 73(2): 122-134.
Palabras clave: ametionina, reversión de NAFLD y terapia nutricional.
https://doi.org/10.37527/2023.73.2.004
Autor para la correspondencia: Rebeca García-Román, PhD, e-mail: [email protected]
Being overweight and obese are risk factors for non-alcoholic fatty liver disease (NAFLD), also known as metabolic dysfunction- associated fatty liver disease (MALFD) (1).In the USA, the prevalence of this disease among Hispanics of Mexican ancestry is 22%, one of the highest (2). Simple steatosis is a benign stage but can be aggravated tonon-alcoholic steatohepatitis (NASH) when it converges with inflammation (3). The primary treatment for NAFLD is diet modification, weightloss (5-10%), and a moderate increase in physical activity (150 - 200 min / week)(4). However, neither weight reduction nor body mass index (BMI) produced by caloric restriction is essential for reducing the content of liver fat that can restore its function (5). Currently, there is no specific diet for the treatment of NAFLD.
Dietary habits are closely associated with the general state of health. Modifying eating habits is a useful tool for treating insulin resistance, metabolic syndrome, dyslipidemia, and hypertension (6). Changes in the composition and quality of diet can influence the clinical course of NAFLD beyond simple caloric restriction. Furthermore, it could positively influence the quality of life of patients with chronic diseases.
Currently, efforts have been made to identify nutrients and micronutrients that favor the reduction of steatosis, as well as its clinical parameters (7). The Mediterranean diet (MetD) is the most prevalent diet in patients with NAFLD. This includes the consumption of fruits, vegetables, olive oil, cereals, fish, and red wine in countries bordering the Mediterranean Sea.
A review of the effect of MetD in patients with NAFLD showed that the main changes are weight loss, decreased glucose levels, improved steatosis, and reduced BMI, cholesterol, and triglycerides (8). However, parameters that characterize liver damage, such as the AST/ALT ratio, are not diminished, and no conclusive data support the use of MetD as a non-pharmacological treatment for NAFLD. A comparison between the MetD Score and the International Diet Quality Index (DQI-I) showed better results in DQI-I than the MetD Score, concluding that the consumption of vegetables, legumes, fruits, and nuts produced a reduction of 50% in the risk of NAFLD (9).
Since the pathogenesis of NAFLD is related to oxidative stress and inflammation (10, 11), it is logical to look for antioxidant agents or compounds involved in the redox system that can be used to overcome this condition. Several studies have used antioxidants such as vitamins E, C, and D, resveratrol, anthocyanin, and quercetin (12); however, many of these studies are still in the experimental stages in animals or have not shown conclusive data in humans, so it is necessary to study other agents or diets in clinical trials that can prevent, attenuate, or reverse NAFLD.
Methionine is a source of methyl groups in methylation reactions, including the generation of the main intracellular antioxidant, glutathione (GSH). Methionine is converted to S-adenosyl methionine (SAM), which is essential as a methyl donor for membrane phospholipids (13). Eighty-five percent of all transmethylation reactions and more than 48% of methionine metabolism occur in the liver (14). Therefore, a lack of SAM produces pathological effects. The action of SAM in NAFLD has been studied in several murine models and cell lines and has even been proposed as a possible therapeutic agent in patients with various liver pathologies (15). To date, no human study has evaluated the effect of a dietary strategy based on the consumption of foods high in methionine for the treatment of NAFLD. The objective of this study was to evaluate a diet containing foods rich in methionine for the treatment of non-alcoholic fatty liver.
We performed a randomized controlled clinical trial in accordance with the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. Patients were recruitedfrom April to October 2015 at a clinical hospital of the Institute of Security and Social Services of State Workers (ISSSTE by its acronym is Spanish), which corresponds to a first- and second-level care medical unit located in the city of Xalapa, Mexico. The inclusion criteria were as follows: NAFLD identified through the fatty liver index (FLI) (16) confirmed by liver ultrasound and age ≥20 years.
Subjects with cirrhosis, Wilson's disease, viral hepatitis B and C, hepatocarcinoma, or other malignancies were excluded.
The experimental group consisted of nutritional therapy administration of foods with a high content of methionine according to the National Nutrient Database for Standard Reference (USDA) (17), and adapted to the consumption and ordinary cost in the Mexican diet. Supervision was carried out through 24-hour reminders diet during a 3-month follow-up period, where the food consumed, and the quantity were recorded. As the daily requirement of methionine for adults is 260-700 mg (18), patients were instructed to combine foods until they achieved a daily intake of at least 700 mg (Table S1, Supplementary Material).
Through an interview, age, level of education, and economic income were inquired.
Two nutritionists performed the diet instructions and a 24-hour reminder. At the end of the three-month follow-up, the food consumed, daily rations, and the monthly average methionine mg in the food were recorded for subsequent analysis. The control group continued the usual traditional diet. The traditional Mexican diet is composed of grains, legumes, and vegetables, whereas specific food items include maize, beans, and chile. Additionally, maize products, fruits, beverages, fish and seafood, meats, sweets, sweeteners, herbs, and condiments may still play an important role in the traditional Mexican dieta (19).
The main outcome variable was NAFLD reversal (FLI< 60). The FLI was calculated using the waist circumference (WC), BMI, gamma-glutamyl transferase (GGT), and triglycerides algorithm, in accordance with Bedogni et al (16):
The secondary variables were ALT, AST, GGT, metabolic syndrome criteria (BMI and waist circumference [WC], glucose, total cholesterol, triglycerides, and blood pressure), average daily consumption of methionine in foods, and health-related quality of life.
Through interviews, age, level of education, income, and quality of life were investigated. Variables such as ALT, AST, GGT, glucose, total cholesterol, and triglycerides were measured using photometric analysis (Cobas c111, Roche, Ltd. Germany). The BMI was calculated using a bioelectrical impedance scale (|ITA model TBF-410 GS). Height and WC were measured using a stadiometer and tape measure. All variables were measured at baseline and at the months follow-up in both the groups.
To evaluate health-related quality of life, the SF-36 questionnaire was used to assess physical function, functional/physical role, functional/emotional role, vitality, mental health, social function, body pain, general health, and health transition. This questionnaire was adapted to the Mexican population (20).
Based on the reported FLI mean differences (- 4.36) (21, 22), and with a confidence level of 95% and a power of 90% test, the specified minimum sample size for each group in our study wasnine subjects. The sample size was calculated using Epidat: Program for epidemiological data analysis, version 4.2 (July 2016). Subjects were assigned to the experimental or control group using random computer numbers. The groups were matched by sex to ensure an equitable distribution and avoid confounding effects of the sex variable. Four blocks were created: men were assigned to the experimental group, women to the experimental group, men to the control group, and women to the control group.
The researcher who generated the random numbers was unaware of the identity of the study subjects. A second investigator blinded assigned the subjects to the groups. However, due to the nature of the intervention, it was not possible to blind the subjects or supervise nutritional therapy concerning the intervention received. The statistically responsible person did not know the experimental or control group.
A per-protocol analysis was performed on subjects who completed follow-up for three months without interruption. Patients who dropped out of the study during follow-up were analyzed by intention-to-treat.
Student's t test was used to compare means, and the chi-square test was used to compare proportions. In the comparison of means at 3 months of follow-up with respect to baseline, a t test for paired groups was used. The frequency of subjects undergoing NAFLD remission at the three-month follow-up was estimated as the cumulative incidence. The comparison of accumulated incidences between groups is presented as relative risk accompanied by a 95% confidence interval. Pearson's correlation coefficient was calculated to measure the relationship between the daily consumption of methionine in food and the biochemical and anthropometric variables. In addition, a multiple linear regression model was performed, considering the FLI and mean daily consumption during the third month of follow-up, metabolic syndrome, and age. Statistical significance was set at p value≤ 0.05. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY.
The Research and Ethics Committee at the study headquarters approved the study protocol. The study subjects provided written informed consent, and all their rights were respected following current national and international regulations. The protocol was registered on the ClinicalTrials.gov platform (ID-number number NCT04450875).
From a sample of 205 patients diagnosed with NAFLD, 84 did not accept to participate (41%), so 121 subjects were recruited for clinical trial, of which 56 were random ly assigned to the experimental group and 65 to control group (Figure 1). There were no significant differences at baseline between the groups concerningsex (pairing criteria), schooling, family income, blood glucose, systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI, WC, total cholesterol, triglycerides, ALT, AST, GGT, and FLI (Table 1). Only the mean of HDL-cholesterol was significantly higher in the experimental group (45.6 vs. 40.3 mg /dL).
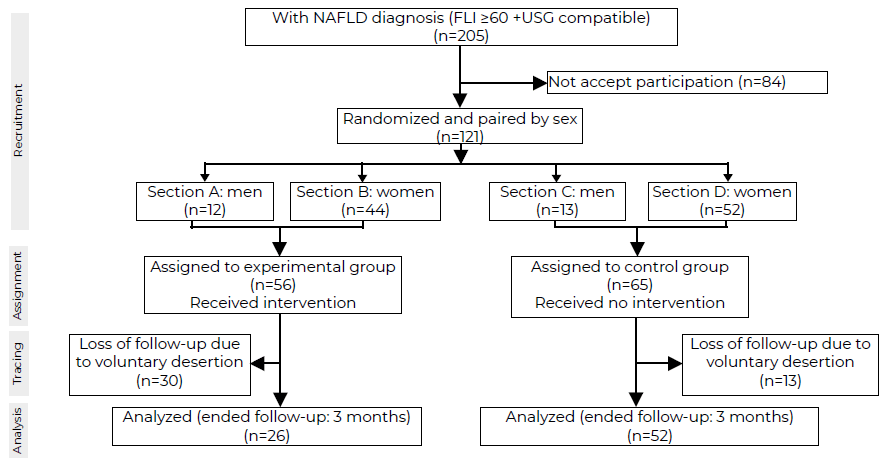
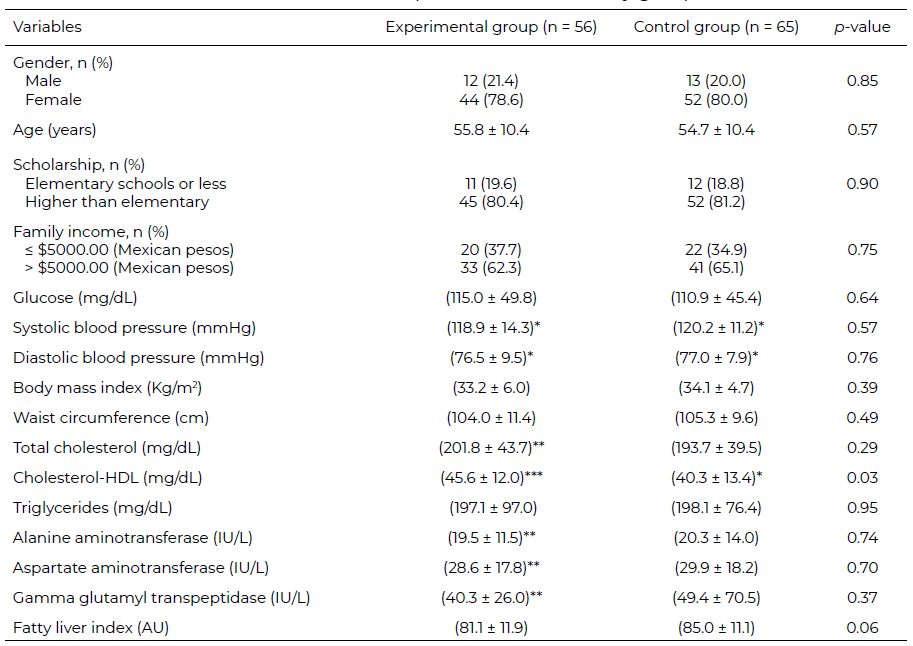
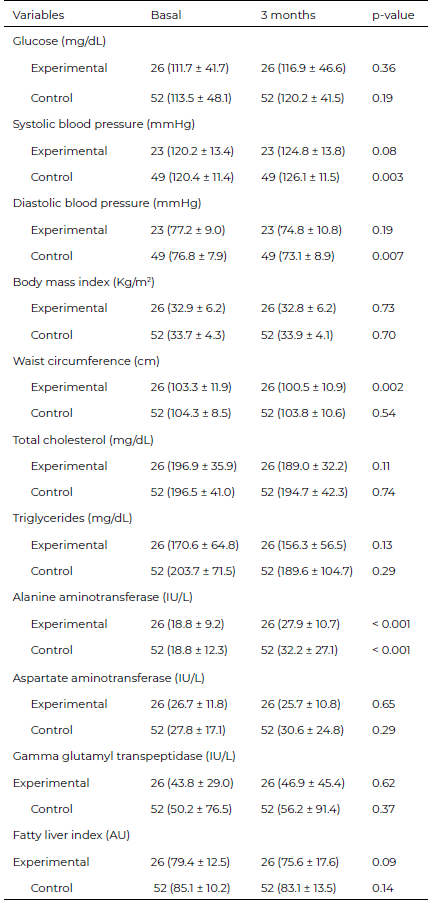
During the study, 30 subjects out of 56 (53.6%) in the experimental group and 13 out of 65 (20.0%) in the control group were lost to follow-up, no significant differences were found in the frequency of sex; instead, the subjects who dropped out were significantly younger than those who remained, this difference was found only in the control group (Supplementary Material, Table S2). Table 2 compares the subjects in the experimental group at baseline with regard to the three-month follow-up; the same was done in the control group. In the experimental group, the mean WC decreased significantly (from 103.3 cm at baseline to 100.5 cm), but this was not observed in the control group. The mean SBP increased significantly in the control group (from 120.4 mmHg to 126.1 mmHg), but not in the experimental group. In contrast, mean DBP decreased significantly only in the control group (76.8 mmHg 73.1 mmHg). Finally, both groups showed a significant increase in the ALT enzyme levels. No statistically significant differences were observed in glucose, BMI, total cholesterol, total triglyceride, AST, and GGT levels. Regarding FLI, both in the experimental and control groups, there was a decrease in the mean score at three months from baseline. Although the difference was greater in the experimental group than in the control group, the differences were not statistically significant.
When the intention of treatment was analyzed, remission was 1.2 times higher in the experimental group than inthe control group.However, when the analysis was performed per protocol, the probability of remission was twice as high in the experimental group;however, neither analysis showed a statistically significant difference (Table 3). Table 4 compares the experimental group vs. control at the months follow-up (intergroup comparison); the FLI was lower in the experimental group (75.6 AU) than inthe control group (83.1 AU), p = 0.04; no significant differences were found between groups in the rest of the variables studied.
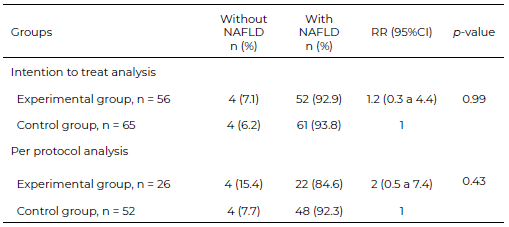
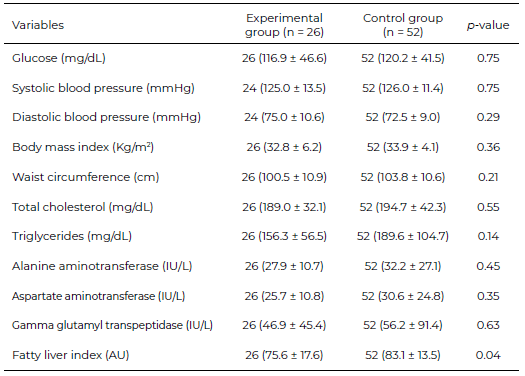
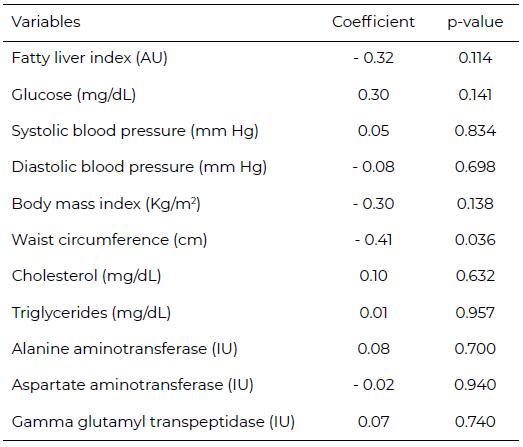
Table 5 shows, the correlation between biochemical and anthropometric variables with the daily average of methionine consumed in food. Waist circumference was the only variable that showed a statistically significant negative correlation (r = - 0.41).
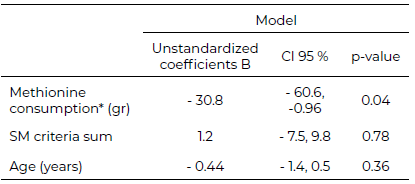
In a multivariate analysis, in whose models the dependent variable was the FLI, and the covariates were the mean daily consumption of methionine in food during the third month of follow-up,the metabolic syndrome (MS) criteria, and age. The only variable significantly associated with the FLI was methionine consumption in food (Table 6).
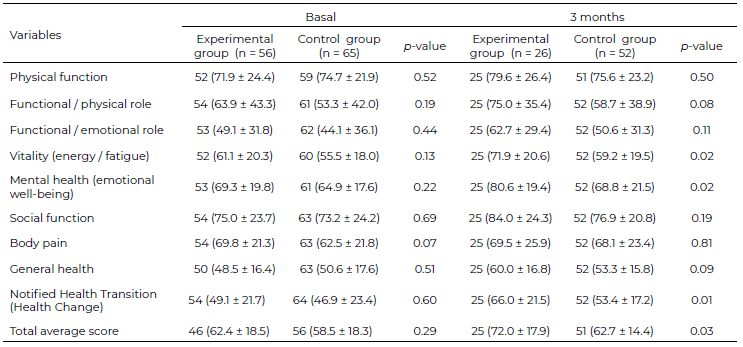
The health-related quality of life measured using the SF-36 questionnaire (version 1.1; authorized use in Mexico) is presented in Table 7. There was no significant difference between the experimental and control groups at baseline (comparison between the groups). However, after three months of follow-up, the mean of the scores of the domains: “vitality,” “mental health,” “reported health transition,” as well as the mean of the total score were significantly higher in the experimental group than in the control group (Table 7). In an analysis within each group that compares the baseline moment with regarding the three months of follow- up (data not shown), the mean score was significantly increased in the experimental group; that is, there was an improvement, in the following domains: "physical function,” "mental health,” “social function,” “general health,” “notified health transition” and in the “total average score.” The control group also improved in the following domains: “notified health transition” and “the total average score”
The therapeutic option with the highest demand for NAFLD treatment is weight loss through caloric restriction. Until now, the diet most investigated for its possible effects on NAFLD was MetD. However, there are also other approaches, such as the intake of micronutrients, including vitamins E and D, in addition to some omega-3 polyunsaturated fatty acids (n3-PUFAs)(23, 24). Many of these investigations,although they provided relevant data, were carried out with small experimental groups of 20 or fewer people and without control groups, which reduces the validity of their results. A clear example is the study with betaine, which only had 10 participants, of whom only 7 completed the 1-year treatment (25). In another study in which polyunsaturated fatty acid omega-3 was administered, only 20 people were included in each experimental group (24). The strength of our study lies in the number of subjects included, since each experimental group exceeded 50, which makes it methodologically more solid and reproducible. The ages of the patients analyzed in some of these studies ranged from 50 to 55 years, which is where the disease occurs most frequently, according to our own results.
In our study, the FLI variable showed a significant decrease of 75.6 in the experimental group compared to 83.1 in the control group at the end of treatment. Other authors in previous studies that used MetD as a tool for treating NAFLD found a similar decrease in liver fat (23, 24). However, the study by Ryan et al, although it decreased liver fat, Ryan et al. did not demonstrate a decrease in AST and ALT levels. The authors suggested that this negative effect could be due to the short treatment duration. Although our results showed similar ALT levels in both groups, this increase was minor in the experimental group comparedto that in the control group. In two previous studies that used betaine (component of the methionine cycle)(25, 26) alone or in combination with glucuronate at doses of 300 mg and 20 g daily; they achieveddiminution of ALT levels. This decrease was most likely due to the long period of administration (1 year) and high doses of the supplements, although only 10 patients completed the treatment. An important aspect to consideris the use of purified supplements in the methionine cycle at very high doses that can have harmful or no effects (27, 28). Our approach focused on the intake of safe whole foods high in methionine, but perhaps the short treatment period did not allow for an improvement in liver enzyme levels.
Few antioxidants have been tested in the context of NAFLD. The antioxidant vitamins C and E administered at a dose of 800-1000 IU/d for periods of 6-24 months, were able to decrease the levels of AST and liver fibrosis in patients with NASH (29, 30). It should also be noted that in the study in which vitamins E and C were used, only 45 patients completed the treatment. Controversially, other studies have revealed that doses above 400 IU/d of vitamin E increase the mortality and risk of prostate cancer (31, 32). Since toxicological effects could occur at high doses, food administration programs must rigorously monitor follow-up for supplementation with antioxidants, which makes the strategy of using nutritional therapies that include adequate proportions of antioxidants in foods more relevant.
In contrast to BMI, WC is strongly associated with visceral fat (33) and is a reflection of the percentage of body fat and the liver. Our results showed a decrease in WC, which was not observed in the control group. It should be noted that the implementation of nutritional therapy based on foods high in methionine without additional intervention was sufficient to decrease the above parameters. Several studies have shown that reducing BMI is not sufficient to decrease liver fat levels. Reductions in liver fat have been achieved regardless of the degree of BMI reduction with a low- fat isocaloric diet (34). Although the duration of our dietary intervention did not exceed three months, it was sufficient to reduce parameters such as WC and FLI. Other investigations observed a similar reduction, but with longer treatments, even in years (35).
The proportion of women in the recruitment phase of our study was higher than men, this may be due to the fact that women tend to have a greater self-care of their health as well as their self-image (36).
HRQL has been widely measured through the SF- 36, whose parameters include physical function, vitality, mental health, and general health. In 2016, the quality of life parameters were examined using the SF-36 instrument in NAFLD patients with or without cirrhosisand compared to the general population. A decrease in SF-36 scoreswas observed in cirrhotic NAFLD patients compared tonon-cirrhotic patients and was well below the general population (37). The course of NAFLD alone causes deterioration in quality of life as perceived by the patient. In our study, comparing the quality of life only in the experimental group before and after nutritional therapy was started, physical function, mental health, social function, and general health showed a significant improvement. To our knowledge, this study is the first to demonstrate the impact of nutritional therapy on the quality of life of patients with NAFLD.
Study limitations. The present clinical trial has limitations in the extrapolation of the results: a high proportion of non-response (41 %); a high frequency in the loss of subject during follow-up (54% and 20% in the groups experimental and control, respectively) and the lack of recording of caloric adequacy, physical activity and additional vitamin supplements. First, clinical trials are not characterized by achieving a strong population representativeness; on the contrary, they seek to select subjects in ideal conditions to demonstrate that the outcome variable results from the intervention.The random assignment to the comparison groups is responsible for reducing the probability of a possible confounding effect of some of the other covariates. Second, no significant differences were found in the distribution by sex between the subjects who were lost to follow-up and those who underwent complete follow-up. In contrast, the control group subjects who dropped out were significantly younger than those who remained. Per protocol and intention-to-treat analyses were performed to assess the degree of influence of the subjects lost to follow- up; in both cases, the results were similar; that is, no significant association was found between a diet rich in methionine and FLI reversal
Our study has methodological strengths that favor the validity of the results: an analysis by protocol and by intention of treat;a control group; the random assignment; the size of the groupsexceeds the estimated minimum sample; parity by sex; a researcher blindly analyzed the information; and a multivariate analysis was performed.
Finally, our study showed preliminary findings that demonstrated the diminution of NAFLDwith nutritiontherapybased on foods rich in methionine.
We want to thank the Fondo Sectorial de Salud, Consejo Nacional de Ciencia y Tecnología (CONACyT), of the Mexican government for supporting our work under Grant number 233533.
The authors have declared that no conflict of interest.
Recibido: 15/02/2023
Aceptado: 03/04/2023